- Research article
- Open access
- Published:
Chemotherapy dose per kilogram lean body mass increased dose-limiting toxicity event in male head and neck cancer with taxane and platinum-based induction therapy
BMC Cancer volume 22, Article number: 1084 (2022)
Abstract
Background
This study aimed to determine whether drug doses per kilogram of lean body mass (LBM) were associated with dose-limiting toxicity (DLT) events in head and neck cancer (HNC) patients.
Methods
This retrospective cohort study included 179 HNC patients who underwent induction chemotherapy (IC) at a medical center from May 1, 2014, to May 31, 2021. HNC patients’ characteristics, tumor factors, IC regimen and dose, laboratory data, and body composition factors, including lean body mass (LBM) and skeletal muscle index (SMI), derived from CT, MRI, or PET scan images and drug dose per kilogram LBM were recorded. Dose-limiting toxicity (DLT) events were regarded as the primary outcome. Multivariate logistic regression was used to establish a novel risk score for DLT events by the abovementioned variables. The above-mentioned risk score was validated in another cohort.
Results
The overall DLT events during the first cycle of IC for 179 HNC patients was 24%. After stratifying by gender, docetaxel per kilogram LBM > 2.52 mg/kg (adjusted odds ratio [aOR]: 3.18; 95% confidence interval [CI], 1.25–8.09), pre-treatment glutamic pyruvic transaminase (GPT) > 40 U/L (aOR, 2.61; 95% CI, 1.03–6.64), and history of chronic liver diseases (aOR, 3.98; 95% CI, 1.03–15.46) were significant variables in male HNC patients. The DLT events risk was categorized by summation of the above-mentioned risk factors for male HNC patients. Three risk groups were stratified by overall event of 17.6%, 25.8%, and 75%. The above-mentioned risk score had an acceptable discriminatory ability in another validation cohort.
Conclusions
Among male HNC patients treated with IC, docetaxel per kilogram LBM more than 2.52 mg/kg, pre-treatment GPT > 40 U/L, and history of chronic liver disease were significant risk factors for DLT events. Identifying high-risk patients could help physicians prevent severe/fatal complications among HNC patients undergoing IC, especially for the male individuals.
Background
Head and neck cancer (HNC) is one of the most common and lethal cancers worldwide [1, 2]. Despite considerable improvements in treatment, such as radical resection, radiotherapy techniques, targeted therapy, and immunotherapy, HNC remains notorious for its high recurrence rates and distant metastasis rates [3, 4]. Recently, induction chemotherapy (IC) has been increasingly utilized. IC could be followed by definitive radiotherapy with or without chemotherapy or surgery during an organ preservation strategy in oropharyngeal, laryngeal, or hypopharyngeal cancer. After the improvements in primary care, an increasing number of promising studies have revealed IC’s protective effects, including decreased distant metastasis rates, improved survival rates, and organ preservation [5, 6].
Treatment-related toxicities, such as severe mucositis, febrile neutropenia (FN), acute kidney injury (AKI), and hyponatremia, are also increasingly being reported [7, 8]. Dose-limiting toxicity (DLT) event could lead to therapy dose reduction or delay and resulted in worse outcomes in turn [9]. Besides traditional factors, like comorbidities, performance status, and impaired renal or hepatic function, body composition factors, and related variables, such as chemotherapy per kilogram lean body mass (LBM) were reported to be associated with DLT [10, 11]. Low skeletal mass or sarcopenia was associated with increased chemotherapy dose-limiting toxicity (DLT) in HNC with chemoradiotherapy [12]. Chemotherapy dose per kilogram LBM had been reported to be significant predictors for toxicity event or DLT in lung cancer and colon cancer [13, 14]. However, its validity in HNC with induction chemotherapy was not explored.
In order to achieve adequate relative dose intensity (> = 80% at least) in chemotherapy, the first step was to identify high-risk populations for DLT and launch prevention protocols, like prophylactic G-CSF injection, more intensive care, or close follow-up [9, 15]. We aimed to perform a comprehensive analysis, consisting of chemotherapy dose per kilogram LBM, body composition factors, patients’ characteristics and tumor factors, laboratory data, etc., and develop a prediction model for DLT events in HNC patients with IC using a cancer registry database and clinical research database at our institution.
Methods
Patient population and treatment
From the Cancer Registry Database of Kaohsiung Veterans General Hospital, 179 HNC patients who underwent taxane and platinum-based IC between May 1, 2014, to May 31, 2021, were identified (Fig. 1). IC was administered if a patient was under suitable conditions and had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2. Our hospital has two main chemotherapy regimens, abbreviated as TP, and TPF. All regimens were administered every three weeks for two to three cycles. Patients who were scheduled for two to three cycles failed to undergo subsequent chemotherapy due to adverse events were also included for analysis. The TP regimen was the most used and consisted of docetaxel (60 mg/m2 on Day 1) and cisplatin (60 mg/m2 on Day 1). The TPF regimen was TP plus 5-fluorouracil (500 mg/m2 as a 4-day continuous infusion). For patients with poor renal function, cisplatin was replaced by carboplatin. After IC, no prophylactic antibiotics were prescribed unless severe toxicity occurred. The patient was re-evaluated using imaging studies to identify their overall response and determine the treatment course after two to three IC cycles.
The independent variables were categorized into three domains. One domain included the patients’ characteristics (age, sex, comorbidities, etc.) and tumor features (sub-sites, clinical TNM stages recommended by the American Joint Committee on Cancer [7th edition before 2018, and 8th edition thereafter] [16, 17], etc.). The second domain also included clinical laboratory data (complete blood cells and differential count, sodium, potassium, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), total bilirubin, albumin, glucose level, blood urea nitrogen, creatinine, etc.). Another domain included body composition measurements, including lean body mass (LBM) and skeletal muscle index (SMI). A third domain had chemotherapy dose and dose per LBM. SMI was derived from a cross-sectional area (CSA) at the third cervical vertebra of the pre-treatment computerized tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) scan images, as previously described [18, 19]. Informed consent was not required due to the use of non-identifiable records. The Ethics Committee of our Institutional Review Board approved the study protocol (KSVGH22-CT1-03).
Body composition measurements and chemotherapy dose per kilogram lean body mass
Body composition was estimated using the method developed by Swartz et al. [20]. The skeletal muscles CSA was measured on the reference slice as the first axial view where all vertebral arcs could be identified, along with the transverse and spinous processes. The measurement was taken by scrolling from the caudal to the cephalad direction on the head-and-neck images. The skeletal muscles CSA was manually determined twice by a single physician (Supplementary Fig. 1). The skeletal muscles CSA at the C3 vertebra (in square centimeters [cm2]) was determined as the sum of separate measurements of the paravertebral and bilateral sternocleidomastoid muscles. The intra-rater reliability correlation coefficient for CSA at C3 was acceptable (0.94; 95% confidence interval [CI], 0.923–0.953) (Supplementary Table 1). The following formulae were also used to calculate CSA, SMI, and LBM:
Chemotherapy dose per kilogram lean body mass was determined as:
Dose-limiting toxicity events and patient follow-up
The dose-limiting toxicity (DLT) events included (1) grade 3 or higher acute hematological adverse events like neutropenia (absolute neutrophil count [ANC] < 1000 with or without fever), anemia (hemoglobin < 8 mg/dL, or with life-threatening consequences), and thrombocytopenia (platelet < 25,000/μL), and non-hematological adverse events, such as acute liver injury (total bilirubin > 1.5×upper limit of normal (ULN); GOT or GPT > 5 × ULN) and acute kidney injury (creatinine > 3× ULN) according to the Common Terminology Criteria for Adverse Events version 5.0 or death after IC (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf), (2) death after chemotherapy, (3) subsequent dose reduction of > = 50% for taxane or platinum, or (4) postponement of subsequent therapy of ≥ 4 days due to bone marrow suppression [14]. DLT events after the first cycle of induction chemotherapy was defined as the study endpoint.
Responses after 2–3 cycles of induction chemotherapy were analyzed according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria by CT/MRI images. Subsequent treatment modality was also reported.
Statistical analyses
SAS version 9.4 (SAS, Inc., Cary, NC) was used to analyze the data. Categorical variables, such as sex, primary tumor site, clinical stage, and comorbidities, were analyzed using Pearson’s chi-square or Fisher’s exact test. Continuous variables, such as age, LBM, SMI, and chemotherapy dose per LBM, were compared using one-way ANOVA. Univariate logistic regression was performed at first, and variable with a P-value less than 0.1 were candidates for multivariate analysis. Multivariate logistic regression with backward stepwise method was applied to determine the significant variables. Rounded number of beta coefficient or summation of risk factors in the final model was used to establish a prediction model. Another cohort composed of 34 HNC treated with taxane and platinum-based induction chemotherapy between June 1, 2021, and May 31, 2022, were recruited in order to validate the above-mentioned risk score. A two-sided P-value < 0.05 was considered statistically significant.
Results
Dose-limiting toxicity events in HNC treated with induction chemotherapy
A total of 179 HNC patients who underwent IC with taxane and platinum were recruited. The mean age was 56.5 ± 8.8 years, and 91.1% were male. The study population consisted of 37 (20.7%) patients with oral cancer, 83 (46.4%) with oropharyngeal cancer, 51 (28.5%) with laryngeal/hypopharyngeal cancer, and 8 (4.5%) with other cancers, such as sinonasal cancer (Table 1). The mean estimated LBM and SMI was 40.2 ± 6.6 kg and 47.9 ± 7.3 cm2/m2, respectively. A large variation of docetaxel per kilogram LBM and cisplatin per kilogram LBM from 1.53 mg/kg to 4.14 mg/kg was observed compared to other body composition factors (Supplementary table 2). DLT events during the first cycle of IC was summarized in Table 2. 43 DLT events (24%) were recorded; 35 patients (19.6%) with grade III-IV adverse events, eight patients with death (4.5%), and nine patients (5%) with the postponement of subsequent chemotherapy.
The association between chemotherapy dose per kilogram LBM and DLT in male and female patients
The distribution of body composition factors, chemotherapy dose per kilogram LBM, and DLT events between male and female patients were summarized in Table 3. Male patients had higher LBM (41.4 kg vs 28.5 kg; P < 0.001), and higher SMI (48.9 cm2/m2 vs 37.4 cm2/m2; P < 0.001). There was no statistical difference between the DLT event rate between male and female patients.
Risk score for DLT events in HNC patients
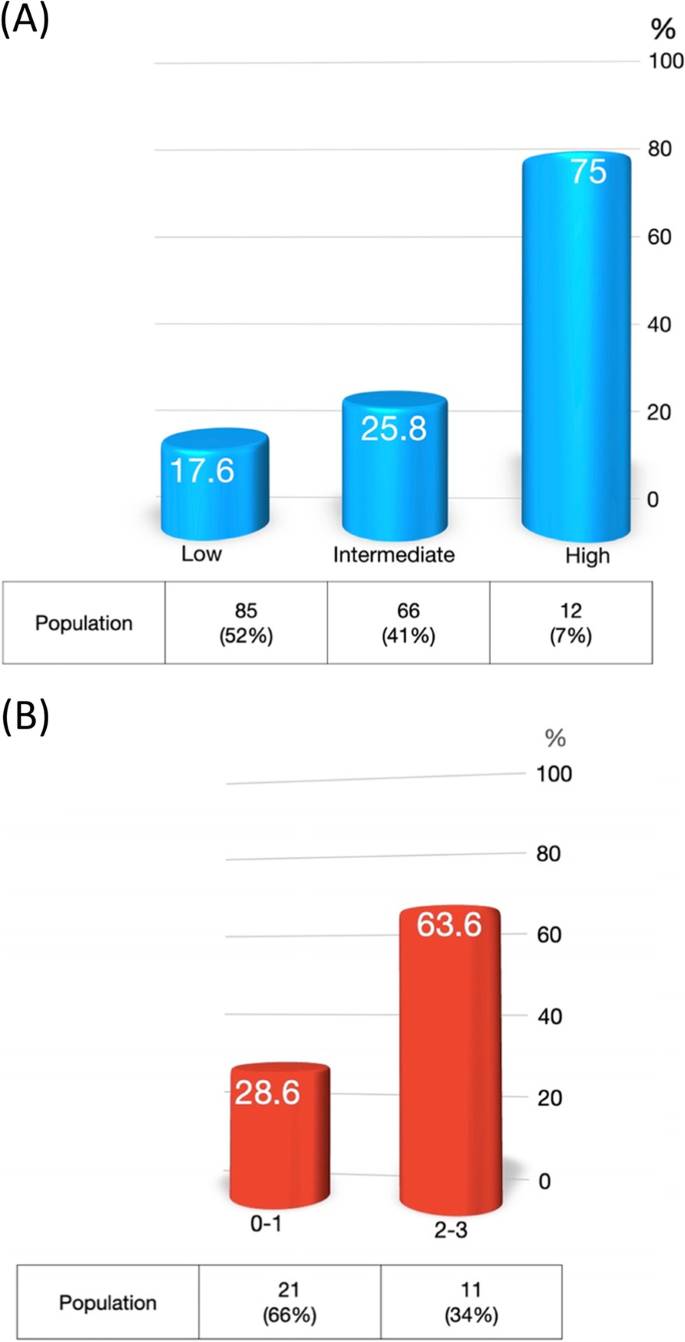
Due to the significant difference of body composition factors and chemotherapy dose per kilogram LBM between male and female patients, we performed univariate and multivariate analysis stratified by gender (Table 4). Due to a large difference of chemotherapy dose per kilogram LBM between male and female patients, different cutoff points were used (Supplementary table 3). Among male HNC patients, docetaxel per kilogram LBM more than 2.52 mg/kg (aOR = 3.18; 95% CI, 1.25–8.09), pre-treatment GPT more than 40 (aOR = 2.61; 95% CI, 1.03–6.64), and history of chronic liver disease (aOR = 3.98; 95% CI, 1.03–15.46) were significant predictors for DLT events in multivariate analysis. The beta coefficient for the abovementioned factors was similar (Supplementary table 4), and these factors could be added together for further risk estimation. There was no significant predictor for female HNC patients. Figure 2A illustrated the association of DLT events by summation of risk factors in male HNC patients. The DLT rate was 17.6% for the low-risk group (with 0 risk factor), 25.8% for the intermediate-risk group (with one risk factor), and 75% for the high-risk group (with two to three factors).
Validation of risk score
Another cohort composed of 34 HNC patients was used to validate the risk score (supplementary table 5). The demographic data between the derivation group and validation group was similar. DLT events developed in 13 (38.2%) patients (supplementary table 6). Due to limited cases in the validation group, the patients were categorized into two groups. Among male HNC patients, the DLT event rates for score 0–1 and 2–3 groups were 28.6% vs. 63.6%, respectively (P = 0.055) (Fig. 2B).
Discussion
This study identified novel predictors of IC toxicities in HNC using a verified cancer registry database. During the first cycle of taxane and platinum-based IC in HNC, up to 24% DLT events were recorded in our series. Docetaxel per kilogram LBM, pre-treatment GPT > 40 U/L, and history of chronic liver disease were strongly associated with DLT events in male patients. The above-mentioned prediction model was validated with another cohort. For male HNC treated with IC, the abovementioned variables should be identified, and high-risk group deserved closer follow-up and care.
Compared to other studies, our research has several strengths. The prediction ability of chemotherapy dose per kilogram LBM was first validated in male HNC patients with taxane and platinum-based induction chemotherapy for DLT events [13]. Although previous literature had used a similar concept, its application for toxicity event prediction was not widespread [14]. Moreover, we have performed a comprehensive analysis for DLT events. Variables like patients’ characteristics, laboratory data, inflammation markers, body composition factors, and chemotherapy dose were all included. In addition, the correctness of our data was validated. The Cancer Registry System in Taiwan is supervised by the Health Promotion Administration of the Ministry of Health and Welfare. They regularly monitor data correctness and implement the accreditation of cancer centers every three years (https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=1061&pid=6071). Most important of all, the above-mentioned prediction model was validated in another cohort with an acceptable discriminatory ability.
Several hematological inflammatory markers, such as the C-reactive protein-to-albumin ratio, modified Glasgow Prognostic Score, neutrophil to lymphocyte ratio, and platelet-to-lymphocyte ratio, have been used to predict IC toxicity in HNC [11]. The plausible mechanism for the toxicity is attributed to poor nutrition, inflammation, and immune suppression. However, a significant drawback was that body mass index (BMI) was the sole body composition factor in their statistical analyses. Besides BMI, body composition factor, like a low SMI or LBM, has been reported to yield adverse effects on chemotherapy dose-limiting toxicity. More specifically, a low SMI is suggested to be related to a chemotherapy dose reduction of ≥ 50% due to neutropenia or nephrotoxicity, a treatment postponement ≥ 4 days in the case of bone marrow suppression, or definitive chemotherapy termination after the first or second chemotherapy cycles in HNC patients receiving definitive chemoradiotherapy [12]. Our previous study has documented the adverse prognostic effects of sarcopenia on overall survival (aHR: 1.74; 95% CI: 1.14–2.67) and disease-specific survival (aHR: 1.67; 95% CI: 1.04–2.67) in oral cancer patients, and the negative effect was even more significant among patients aged < 60 years [18].
Our study revealed that the chemotherapy dose per kilogram LBM, like docetaxel per kilogram LBM more than 2.52 mg/kg could be a significant toxicity predictor in male HNC patients. The primary toxicity mechanism might be chemotherapy overdose in specific populations, such as in sarcopenia or myopenia [21]. Most chemotherapy dosages are determined by BSA, while some have a fixed or capped dose (e.g., carboplatin). Given the BSA-based dose calculation formulae, patients having a similar BSA but with a lower LBM were treated with a higher dose of chemotherapy per LBM. This phenomenon was more prominent in docetaxel than cisplatin cases, which was expected from a pharmacokinetic perspective. Increased adipose tissue denotes a higher distribution for and uptake of lipid-soluble drugs, such as docetaxel, and a lower distribution for water-soluble drugs, such as cisplatin [22,23,24]. This difference explains why patients with a smaller muscle proportion and a higher adipose tissue proportion were more prone to docetaxel toxicities, as both the protective effect of muscle and the negative effect of excess adipose tissue are contributory [25]. Due to limited number of female patients and events, the prediction model was not developed.
Toxicities are sometimes indicative of adequate chemotherapy dose, which may be associated with better outcomes in solid tumor cancers, such as gastric cancer, non-small cell lung cancer, and prostate cancer [26,27,28]. However, toxicities could also result in postponement or termination of chemotherapy, resulting in worse outcomes [12]. In our study, HNC patients with significant toxicities during induction chemotherapy were not associated with better response rates (58.8% in patients without toxicity event vs. 50% in patients with toxicity event, P = 0.389; Supplementary table 7). The benefit of the toxicity event, a proxy of adequate chemotherapy dose per cycle, might be offset by insufficient relative dose intensity (RDI) [9]. Using our risk score, prophylactic protocol, like granulocyte-colony stimulating factor injection or intensive care, could be applied to the high-risk group in order to prevent DLT events and achieve acceptable RDI.
Our study has several limitations. First, we performed a retrospective study with the abovementioned data extracted from our electronic medical records. Although most laboratory data have been rechecked within one week of IC, some variations still existed. Second, this retrospective study was designed to find new predictors instead of creating a new formula for chemotherapy dose modifications in clinical scenarios. Chemotherapy dose modifications require further large-scale, high-quality observational studies and clinical trials, such as cabazitaxel dose modification in prostate cancer [29]. Third, the SMI or CSA at C3 was derived from head and neck CT, MRI, or PET scan images, rendering the possibility of inaccurate estimates. Nevertheless, previous literature has validated the interchangeability of CT- or MRI-derived imaging biomarkers of SMI [19]. Fourth, the DLT event rate was higher in the validation cohort. The difference was due to missing data about DLT events before 2021. We launched a quality improvement for adverse effect monitoring in HNC with chemotherapy since 2021. Thereafter, the records of DLT events were accurate. Fifth, a large variation of body composition factors and chemotherapy per kilogram LBM between the male and female patients were noted. Stratified analysis was performed, and risk score for male HNC patients alone was established due to unequal sample size between male and female patients. Finally, the prediction model for male HNC patients was validated in another cohort with an acceptable discriminatory ability. It deserved another HNC cohort in western countries for external validation and generalization.
Conclusions
High docetaxel per kilogram LBM (> 2.52 mg/kg), pre-treatment GPT > 40 U/L, and history of chronic liver disease were significant risk factors for DLT events in male HNC patients treated with taxane and platinum-based induction chemotherapy. Before an adjusted chemotherapy dose through LBM or other body composition factors becomes available, identifying high-risk patients could help physicians in clinical care, prevent severe/fatal complications and improve the relative dose intensity of chemotherapy among male HNC patients treated with induction chemotherapy.
Availability of data and materials
The datasets generated and analyzed of the current study are available from the corresponding author on reasonable request after the approval of our IRB.
Abbreviations
- IC:
-
Induction chemotherapy
- FN:
-
Febrile neutropenia
- AKI:
-
Acute kidney injury
- LBM:
-
Lean body mass
- DLT:
-
Dose-limiting toxicity
- HNC:
-
Head and neck cancer
- SMI:
-
Skeletal muscle index
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- GOT:
-
Glutamic oxaloacetic transaminase
- GPT:
-
Glutamic pyruvic transaminase
- CSA:
-
Cross-sectional area
- CT:
-
Computerized tomography
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- ANC:
-
Absolute neutrophil count
- ULN:
-
Upper limit of normal
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28.
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27.
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381:1124–35.
Mezi S, Pomati G, Botticelli A, Roberto M, Cerbelli B, Cirillo A, et al. Induction chemotherapy in nonlaryngeal human papilloma virus-negative high-risk head and neck cancer: a real-world experience. Anticancer Drugs. 2020;31:1074–83.
Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14:257–64.
Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–43.
Nielson CM, Bylsma LC, Fryzek JP, Saad HA, Crawford J. Relative dose intensity of chemotherapy and survival in patients with advanced stage solid tumor cancer: a systematic review and meta-analysis. Oncologist. 2021;26:e1609–18.
Bernadach M, Lapeyre M, Dillies AF, Miroir J, Casile M, Moreau J, et al. Predictive factors of toxicity of TPF induction chemotherapy for locally advanced head and neck cancers. BMC Cancer. 2021;21:360.
Mikoshiba T, Ozawa H, Saito S, Ikari Y, Nakahara N, Ito F, et al. Usefulness of hematological inflammatory markers in predicting severe side-effects from induction chemotherapy in head and neck cancer patients. Anticancer Res. 2019;39:3059–65.
Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017;71:26–33.
Sjøblom B, Benth J, Grønberg BH, Baracos VE, Sawyer MB, Fløtten Ø, et al. Drug dose per kilogram lean body mass predicts hematologic toxicity from carboplatin-doublet chemotherapy in advanced non-small-cell lung cancer. Clin Lung Cancer. 2017;18:e129–36.
Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–8.
Aagaard T, Roen A, Reekie J, Daugaard G, Brown PN, Specht L, et al. Development and validation of a risk score for febrile neutropenia after chemotherapy in patients with cancer: the FENCE score. JNCI Cancer Spectr. 2018;2:pky053.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–37.
Lin SC, Lin YS, Kang BH, Yin CH, Chang KP, Chi CC, et al. Sarcopenia results in poor survival rates in oral cavity cancer patients. Clin Otolaryngol. 2020;45:327–33.
Faron A, Sprinkart AM, Kuetting DLR, Feisst A, Isaak A, Endler C, et al. Body composition analysis using CT and MRI: intra-individual intermodal comparison of muscle mass and myosteatosis. Sci Rep. 2020;10:11765.
Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KM, de Bree R, et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016;62:28–33.
Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29:ii1–9.
Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857–66.
Cisplatin. Lexicomp. Updated Sep 21 2021. Accesed 29 Sep 2021.
Decetaxel. Lexicomp. Updated Sep 20 2021. Accessed 29 Sep 2021.
Gouérant S, Leheurteur M, Chaker M, Modzelewski R, Rigal O, Veyret C, et al. A higher body mass index and fat mass are factors predictive of docetaxel dose intensity. Anticancer Res. 2013;33:5655–62.
Di Maio M, Gridelli C, Gallo C, Shepherd F, Piantedosi FV, Cigolari S, et al. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 2005;6:669–77.
Shitara K, Matsuo K, Takahari D, Yokota T, Shibata T, Ura T, et al. Neutropenia as a prognostic factor in advanced gastric cancer patients undergoing second-line chemotherapy with weekly paclitaxel. Ann Oncol. 2010;21:2403–9.
Kosaka T, Shinojima T, Morita S, Oya M. Prognostic significance of grade 3/4 neutropenia in Japanese prostate cancer patients treated with cabazitaxel. Cancer Sci. 2018;109:1570–5.
Eisenberger M, Hardy-Bessard AC, Kim CS, Géczi L, Ford D, Mourey L, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m(2)) and the currently approved dose (25 mg/m(2)) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198–206.
Acknowledgements
The authors thank the personnel at the Cancer Center, Department of Medical Education and Research, and Research Center of Medical Informatics of Kaohsiung Veterans General Hospital for providing information in response to inquiries and assistance in the data processing.
Funding
This study was partly supported by Kaohsiung Veterans General Hospital (Grant No.: KSVGH 110–139). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
CJH and CCL came up with the concept and design of this analysis. BHK and KMC collected the data with assistance from YYK. CCL and CHY performed statistical analyses. CJH wrote the first draft of the manuscript with assistance from CCL, BHK, KMC, and YYK. All authors were involved in the critical revision of the manuscript and gave their approval for the final version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of the Institutional Review Board of Kaohsiung Veterans General Hospital approved the study protocol (KSVGH22-CT1-03). Because we did not use human participants or personally identifiable records in our analysis, the Institutional Review Board of Kaohsiung Veterans General Hospital decided to waive the informed consent for this study.
Consent for publication
Not applicable.
Competing interests
The authors declared they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary table 1.
Intra-class correlation for different images at initial recruitment. Supplementary table 2. Variation of body composition factors and chemotherapy dose. Supplementary table 3. Cutoff points of chemotherapy per kilogram lean body mass for male and female head and neck patients. Supplementary table 4. Beta coefficient of independent predictors for DLT events in male head and neck cancer patients. Supplementary table 5. Demographic data of the validation cohort (n = 34). Supplementary table 6. Frequency of toxicity events after the first cycle of induction chemotherapy in the validation cohort (n = 34). Supplementary table 7. Toxicity event during the 1st cycle and response rate (n=125).
Additional file 2: Supplementary Figure 1.
Example of skeletal muscle measurements at the third cervical vertebral (C3) level on axial neck CT (A) and MRI (B) images. The paravertebral muscles are shown in green. The left sternocleidomastoid muscle is delineated in red, and the right sternocleidomastoid muscle in yellow.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hung, CJ., Kang, BH., Chang, KM. et al. Chemotherapy dose per kilogram lean body mass increased dose-limiting toxicity event in male head and neck cancer with taxane and platinum-based induction therapy. BMC Cancer 22, 1084 (2022). https://doi.org/10.1186/s12885-022-10152-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10152-y