- Research
- Open access
- Published:
Genomic profiling informs therapies and prognosis for patients with hepatocellular carcinoma in clinical practice
BMC Cancer volume 24, Article number: 673 (2024)
Abstract
Hepatocellular carcinoma (HCC) genomic research has discovered actionable genetic changes that might guide treatment decisions and clinical trials. Nonetheless, due to a lack of large-scale multicenter clinical validation, these putative targets have not been converted into patient survival advantages. So, it's crucial to ascertain whether genetic analysis is clinically feasible, useful, and whether it can be advantageous for patients. We sequenced tumour tissue and blood samples (as normal controls) from 111 Chinese HCC patients at Qingdao University Hospital using the 508-gene panel and the 688-gene panel, respectively. Approximately 95% of patients had gene variations related to targeted treatment, with 50% having clinically actionable mutations that offered significant information for targeted therapy. Immune cell infiltration was enhanced in individuals with TP53 mutations but decreased in patients with CTNNB1 and KMT2D mutations. More notably, we discovered that SPEN, EPPK1, and BRCA2 mutations were related to decreased median overall survival, although MUC16 mutations were not. Furthermore, we found mutant MUC16 as an independent protective factor for the prognosis of HCC patients after curative hepatectomy. In conclusion, this study connects genetic abnormalities to clinical practice and potentially identifies individuals with poor prognoses who may benefit from targeted treatment or immunotherapy.
Introduction
Hepatocellular carcinoma (HCC) is a malignant and high heterogeneity tumour originating from the liver. It is the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide in 2020 [1]. The incidence and mortality of HCC in China account for 45.3% and 47.1% of the world, respectively. The 5-year overall survival rate is currently only 14.1% [2]. The major risk factor for HCC is shifting from viral and alcoholic liver disease to obesity, type 2 diabetes, and nonalcoholic fatty liver disease [3]. The molecular pathogenesis of HCC involves the dysregulation of multiple signalling pathways, including Wnt/ß-Catenin, RAS/MAPK, PI3K/AKT/mTOR, TP53/cell cycle, IGFR, and MET, which is related to point mutations, copy number variations, epigenetic alternations, tumour suppressor inactivation and so on [4]. Hopefully, these discoveries will enable us to identify biomarkers for foretelling prognosis or responses to therapy.
The landscape of genetic alterations in HCC has a clear delineation, including the most prevalent mutations affecting TERT promoter (60%) [5], TP53 (12–48%) [6,7,8], and CTNNB1 (11–37%) [9]. The genetic alterations provide potential targets for treatment planning and prognostic assessment of HCC. About 25% of patients with HCC were detected potentially actionable mutations [10]. Sorafenib inhibits tumour growth and angiogenesis by targeting the RAF/MEK/ERK pathway and receptor tyrosine kinases [11]. PRI-724, a specific inhibitor targeting β-catenin, can be used to address HCC due to CTNNB1 mutation [12]. HCC caused by TERT promoter mutation can be intervened by using targeted drugs such as GX301, Imtelstat, and GV1001 [13]. Regarding prognostic markers, ARID1A, MLL, [14] LRP1B, and TP53 mutations, particularly the hotspot mutations R249S and V157F, are associated with poor prognosis for patients with HCC [15, 16]. Song et al. found that TSC2 mutations were independently associated with early recurrence in HCC patients who underwent hepatectomy [17]. Nonetheless, these potential targets are yet to be translated into the actual survival benefits of patients due to the low mutation rates of most driver genes, no targeted drugs for oncogenic mutations, and the lack of large-scale multicenter clinical validation [13].
This study employed multigene sequencing panels targeting cancer driver genes involving key deregulated pathways in HCC, 175 drug-targeted genes, 23 immunotherapy-related genes, and 18 chemotherapy-related genes. Based on the real-world evidence from 111 patients with HCC, we aimed to determine the clinical viability and utility of genome analysis and whether patients can benefit from genomic profiling. Moreover, we identified mutations in four genes associated with survival, mutations in three other genes related to immune infiltration, and 292 novel potentially pathogenic mutations that could serve as potential targets for treatment decisions and prognostic assessment.
Materials and methods
Patient selection and clinical data collection
This is a retrospective study. We screened 111 HCC patients with somatic mutations detected by targeted-capture sequencing. They were treated at the Affiliated Hospital of Qingdao University between October 2015 and November 2020. The follow-up was conducted up to January 15, 2022. Postoperative histopathological examinations confirmed clinical diagnoses. Clinicians gathered clinical data on the progression of the condition (Table S1). All patients were treated surgically. The extent of surgical resection is shown in Table S1. The study was authorized by the Ethics Committee of the Affiliated Hospital of Qingdao University (approval no. QYFYWZLL27327). The informed consent form was offered and signed by each patient. The experiment complied with the official key recommendations of the National Health and Family Planning Commission of China.
DNA extraction, library construction and sequencing
DNA was isolated from tumour tissue samples and whole blood samples (as normal controls) by QIAamp Fast DNA Tissue Kit and QIAamp DNA Blood mini Kit (QIAGEN), respectively. The concentration of DNA was determined using qubit fluorometry, and the integrity and purity were evaluated using agarose gel electrophoresis and the Qubit 2.0 fluorimeter (Thermo Fisher, USA). The targeted DNA sequence was then enriched and captured by two custom sequence capture probes (Nimblegen, USA) that targeted 7708 exons of 508 cancer-related genes and 10,176 exons of 688 cancer-related genes, respectively. Sequencing was performed on the MGISeq-2000 platform with a coverage depth of 1000 × for tumour tissue and 400 × for blood (MGI, Shenzhen, China).
The specific target gene list is in Table S2. There are 850 targeted genes captured by 688 and 508-gene panels, including 345 shared genes, 343 genes specific in the 688-gene panel, and 163 genes specific in the 508-gene panel. In the 508-gene panel, there were 135 genes involved in tumour signalling pathways, 89 associated with targeted therapy, and 16 associated with immunotherapy, 12 of which were associated with both targeted therapy and immunotherapy. The 688-gene panel included 452 genes involved in tumour signalling pathways, 11 associated with chemotherapy, 165 associated with targeted therapy, and 22 associated with immunotherapy, 15 of which involved both targeted therapy and immunotherapy.
Sequencing data analysis
SOAPnuke [18] was used to remove adapters and filter low-quality reads after obtaining raw sequencing data. Using bwa-mem2 (https://github.com/bwa-mem2/bwa-mem2) [19], clean reads were mapped to the human reference genome (hg38). GATK (v 4.1.9.0) [20] was used to eliminate duplicates, identify somatic variants, and filter variants. The assessment of clinical importance and the prediction of the functional impact of sequence variants were done using ANNOVAR (http://www.openbioinformatics.org/annovar/) [21]. Somatic variants were filtered based on the following criteria: i) variants with allele depth < 10 were excluded; ii) variants with allele frequencies < 0.1 were excluded; and iii) variants with population frequencies > 1% were excluded from the further investigation based on the Exome Aggregation Consortium dataset (ExAC http://exac.broadinstitute.org), 1000 Genomes Project (http://www.1000genomes.org/) [22]. ESP6500SI-V2 and avsnp150 databases. Additionally, actionable mutations were identified using OncoKB (http://oncokb.org) [23]. HCC driver genes were identified by IntOGen [24]. TIMER (https://timer.comp-genomics.org/) [25] database offered tumour immune infiltration analysis.
Mutation statistics and visualization
Detailed information about mutations, including their features, distribution, and enrichment in oncogenic signalling pathways, was compiled and visualized using the R package maftools (version 2.8.05) [26]. We measured overall survival (OS) from the date of the first clinic visit to the last follow-up or death. Survival analysis was visualized using the R package survival (version 3.3.1) and survminer (version 0.4.9), using R package "jskm" to make landmark analysis. We used the Oviz-Bio platform to landscape the mutation type, mutated gene, mutation frequency, and clinical data about the patient [27]. Associations between driver genes and clinical features and the difference between the rates of affected cases in the TCGA cohort and this cohort were investigated using Fisher's exact test or the χ2 test. P less than 0.05 was deemed significant.
Results
Clinical characteristics of the patients with HCC
One hundred and eleven patients with HCC were included in this study (17 in female, 92 in male and two unknown). The median age was 53.5 years (range 33–78). According to TNM staging, the majority of patients (44.14%, 49/111) were in stage T1b. Over 40% of patients had small lesions with 2-5 cm tumour diameters, while 14.41% had large lesions with diameters greater than 10 cm. Lymph node metastasis was common in HCC and a key step in tumour metastasis. In our study, over one-quarter of patients developed lymph node metastasis. Furthermore, 30 patients (27.03%) experienced relapses, with the most common site of recurrence being intrahepatic (50%, 15/30). Over 85% of patients had one or more risk factors, including 88 patients with hepatitis B virus infection, 31 drinkers, and 17 with diabetes.
Concerning clinical indicators, the ASL/ALT ratios in serum were less than 0.8 in 29 patients and greater than 1.5 in 14 patients. The AFP level of 56 patients was above 25 ng/ml, and the CA19-9 level of 25 patients was more than or equal to 37 U/ml. In addition, most patients had normal CEA and CA125 levels (Table 1 and Table S1).
The spectrum of somatic mutations in genes
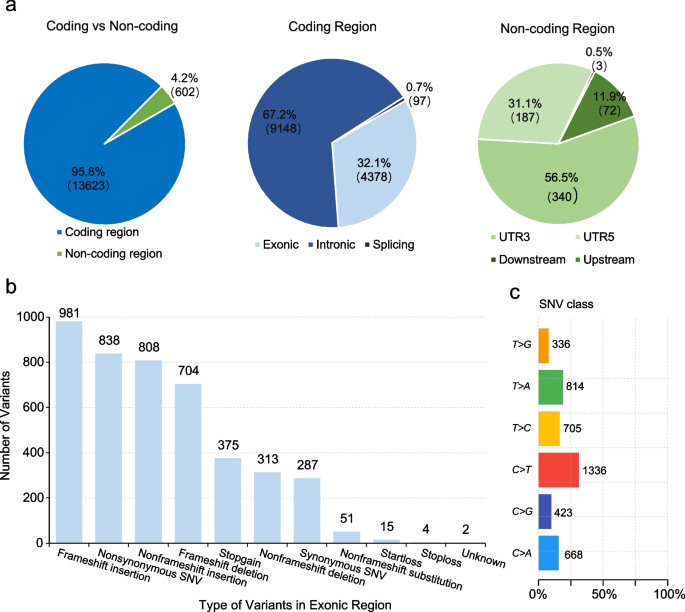
Of these 111 patients, 86 and 25 were detected mutations in 688 genes and 508 genes, respectively. In total, we detected 14,225 somatic mutations in all patients, including 1,125 SNVs, 1,789 insertions, and 1,017 deletions. Most mutations were located in the coding region, 32.1% in exonic regions, and 0.7% in splicing regions (Fig. 1a). The two most common types of mutations were frameshift insertion and nonsynonymous SNV (Fig. 1b). For SNV, C > T was the major mutant form (Fig. 1c). In addition, there were 287 synonymous mutations, 9,148 variants in the intronic region, and 602 variants in the non-coding region, which were filtered out in the following analysis.
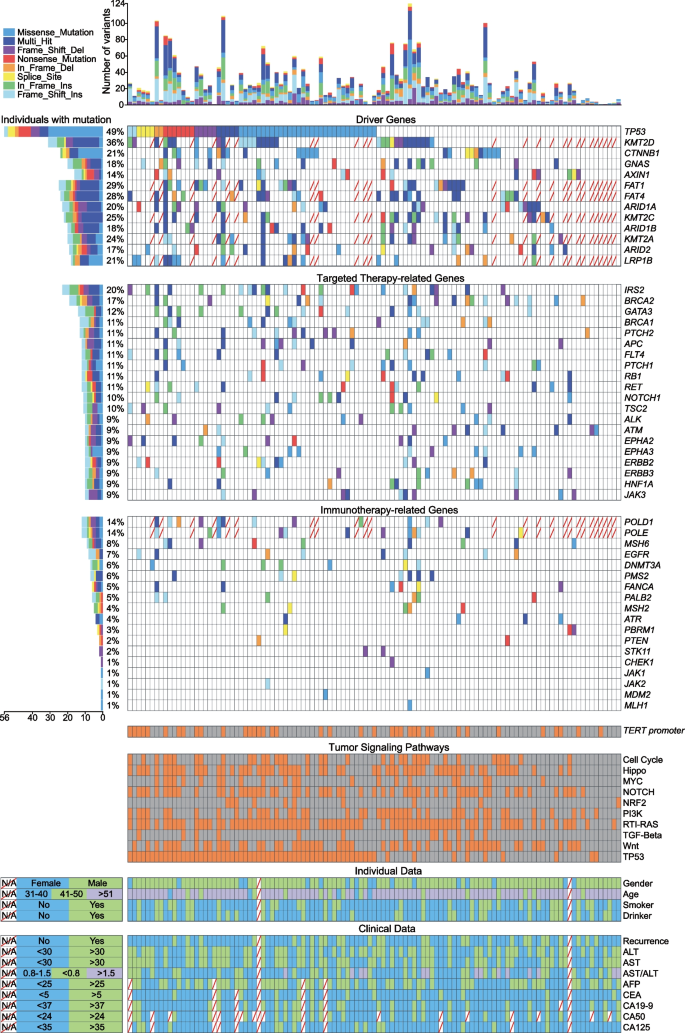
We identified 57 driver genes mutated in 111 patients in this study. About 34% (38/111) of patients harboured TERT promoter mutations. The frequently mutated driver genes were TP53 (50.45%, 56/111), KMT2D (36.05%, 31/86), FAT1 (30.23%, 26/86), FAT4 (29.07%, 25/86), and KMT2C (26.74%, 23/86; Fig. 2). The P53 structural domain was affected most frequently (Fig. 3a). As driver genes, TP53 and CTNNB1 had no interactions with other genes, while Histone-lysine N-methyltransferase 2 (KMT2) family genes had a synergistic effect with the FAT gene family, ARID1A/B, and GNAS (Fig. 3b). Except for these driver genes, the top five high-frequency mutation genes were MUC16 (56.98%, 49/86), APOB (52.33%, 45/86), ZFHX4 (39.53%, 34/86), FAT3 (26.13%, 29/111), and EPPK1 (22.52%, 25/111; Figure S1). Epigenetic modifiers, such as ARID1A (21.62%, 24/111), ARID2 (17.12%, 19/111), and MLL genes, were also recurrently altered.
The landscape of frequently mutated genes of liver cancer. Significantly mutated genes in 111 patients. Above, the histogram shows the number of variants of each patient. Left, the percentages of patients with mutations. Diagonally indicated the information that was not available. Different colours correspond to different types of mutations. Variants annotated as Multhit_Hit are those genes that are mutated more than once in the same sample
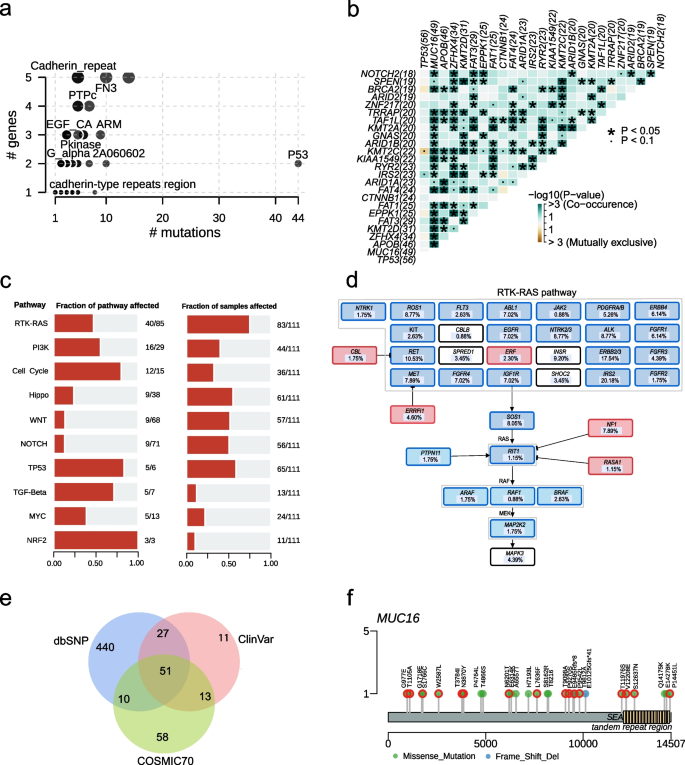
Clinical implications of mutations and domain and pathway enrichment analysis. a Frequently mutated Pfam protein domains in liver cancer. The bubble size is in proportion to the number of genes containing prominent display domains. b Somatic gene interactions. c Enrichment of known oncogenic signalling pathways. d Mutated genes in the RTK-RAS pathway. Tumor suppressor genes are in red, and oncogenes are in blue. e Venn plot of database-registered variants. f Location of the MUC16 mutations schematic. Red circles highlight novel missense mutations
The spectrum of somatic mutations in driver pathways
RTK-RAS (72.81%), TP53 (57.01%), Hippo (53.51%), Wnt/ß-Catenin (50%), NOTCH (49.12%), PI3K (38.60%), and Cell Cycle (31.58%) were activated frequently (Figs. 3c and S1). We found 40 mutated genes involved in the RTK-RAS oncogenic signalling pathway, with five of the mutated genes being tumour suppressors and 35 being oncogenes. Oncogenes IRS2 (20.72%, 23/111), RET (10.81%, 12/111), and tumour suppressor genes NF1 (8.11%, 9/111) frequently mutated in the RTK-RAS pathway (Fig. 3d). The mutations in KMT2D, CTNNB1 (21.62%, 24/111), GNAS (18.92%, 21/111), and AXIN1 (14.41%, 16/111) affected Wnt/ß-Catenin pathway and mutations in TP53, ATM (9.01%, 10/111), RB1 (11.71%, 13/111), CDKN2A (8.11%, 9/111), and CDKN1A (5.41%, 6/111) altered cell cycle control (Figure S2). The oxidative stress pathway was altered in 9.65% of patients with mutations in NFE2L2, KEAP1, and CUL3.
Clinical implications of mutations
We used the CLINVAR, dbSNP, COSMIC, and OncoKB databases to analyze the clinical significance of mutations. In our study, 528, 132, and 102 functional and meaningful variants have been registered in the dbSNP, CLINVAR, and COSMIC databases, respectively (Fig. 3e). More importantly, 92.98% of patients had variants in targeted therapy-related genes (Fig. 2). Among them, 110 variants of 20 genes in 55 patients were reported as drug targets. In other words, 49.55% (55/111) of patients in this study had potentially actionable genomic alterations that required further clinical trials for HCC. For example, frameshift indels and stopain mutations in ARID1A and TSC1/2 were the targets of EZH2 inhibitors (Tazemetostat and GSK126) and mTOR inhibitors (ABI-009 and Everolimus), respectively. The nonsynonymous SNV, G3145C, in exon 21 of PIK3CA was the target of PIK3 inhibitors (Table S3). According to the follow-up results, 15 patients received targeted therapy, immunotherapy, and/or chemotherapy due to tumour recurrence (see Table S1 for treatment options). Three patients were found to carry TP53 mutations associated with sorafenib resistance. This provides evidence for the ultimate selection of lenvatinib. One patient who received sorafenib possessed CCND1 mutation that can result in sensitivity to sorafenib. Moreover, genotype CT of rs11598702 in NT5C2 suggested that one patient with this genotype may have a lower risk of toxic side effects with the use of gemcitabine. Ultimately, this patient also received chemotherapy with gemcitabine. The genetic testing results provided follow-up medication reference evidence for one-third of these 15 patients.
Three gene mutations have been found to be associated with immune infiltration. Based on the TIMER database, patients harbouring TP53 mutations had higher levels of B cells (P = 0.039) and macrophages (P = 0.023; Figure S3). In contrast, patients harboring CTNNB1 and KMT2D mutations had lower levels of CD8+ T cells (P = 0.003 for CTNNB1; P = 0.004 for KMT2D), macrophages (P < 0.001; P = 0.004), neutrophils (P < 0.001; P = 0.002) and dendritic cells (P = 0.004; P = 0.007).
We performed pathogenic mutation prediction using 21 algorithms. For the 631 novel variants, at least five algorithms predicted that 292 variants were deleterious, of which we detected the most novel pathogenic mutations in MUC16, following DNMT3A, UPF1, COL11A1, and BIRC3 (Tables S4 and S5). The variants in MUC16 included 26 missense mutations and two frameshift deletions, of which 57.14% were novel pathogenic mutations. Most novel pathogenic mutations were located outside the SEA and tandem repeat region structural domains (Fig. 3f).
Prognostic implications of genomic and clinical features
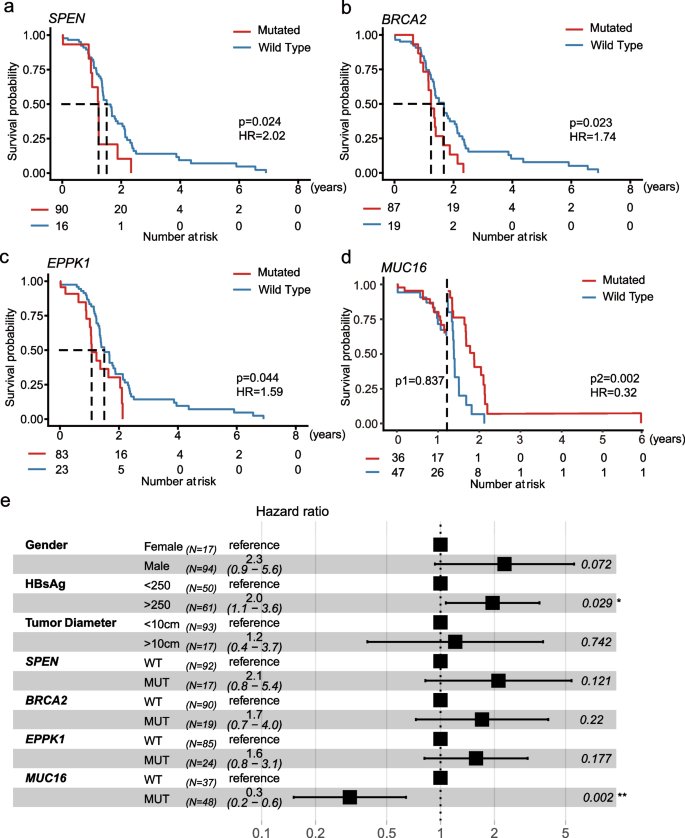
We compared survival between patients with or without mutations in genes. The median follow-up of 111 patients was 14.8 (IQR 0.1–84.0) months. We explored the relationship between genes with mutation frequencies greater than 15% and survival and found that alterations in four genes correlated with a poor or good prognosis. Patients harboring SPEN (18.3 vs. 15.0 months; Log-rank test, P = 0.024; Fig. 4a), BRCA2 (20.3 vs. 15.1[months; P = 0.023; Fig. 4b), and EPPK1 (18.3 vs.13.1 months; P = 0.044; Fig. 4c) mutations had a shorter OS, while patients harboring MUC16 (18.7 vs. 15 months; P = 0.002, after landmark; Fig. 4d) mutations had a longer OS. Mutations in BRCA2 (HR = 1.74), EPPK1 (HR = 1.59), and SPEN (HR = 2.02) were risk factors for patients with HCC, while MUC16 mutation (HR 0.32) was a protective factor.
Moreover, we conducted univariate analysis considering clinical factors (e.g., gender, age, tumour size, HBsAg) and genes with mutation frequencies > 15%. Univariate analysis revealed that SPEN (P = 0.028), BRCA2 (P = 0.026), EPPK1 (P = 0.047), and MUC16 (P = 0.022) mutations were associated with the prognosis of HCC after hepatectomy. No clinical factor was found to be associated with prognosis. Next, we included the above four genes, as well as clinical factors (i.e., gender, HBsAg, and tumor size) in our multivariable regression analysis based on clinical significance and previous literature research findings. Positive HBsAg (HR = 2.3, 95% CI 1.2–4.6) was a risk factor for the prognosis of HCC patients after curative hepatectomy. Otherwise, mutant MUC16 (HR = 0.2, 95% CI 0.1–0.5) was a prognostic protective factor (Fig. 4e).
Associations between driver genes and clinical characteristics
We explored the relationship between driver genes and clinical characteristics. LRP1B mutations were more common in smokers (55.88% vs 44.12%; χ2 test, P = 0.03). Also, LRP1B mutations appeared to be associated with tumor diameter, which was more likely to be greater than 10 cm in patients with LRP1B mutations (68.75% vs. 31.25%; P = 0.01; Table S6).
Discussion
Based on the real-world evidence from 111 Chinese patients, this study enhanced our comprehension of genome analysis's clinical viability and utility in HCC. Approximately 95% of patients had mutations in driver genes and/or pathways in HCC and 48.25% potentially actionable alterations, which yielded valuable information for targeted therapy or immunotherapy. TP53, CTNNB1, and KMT2D mutations were related to immune cell infiltration. SPEN, EPPK1, BRCA2, and MUC16 mutations were associated with OS. More importantly, we identified mutant MUC16 as an independent protective factor for the prognosis of HCC patients after curative hepatectomy.
We revealed hotspot mutations in 111 Chinese patients with HCC, and the genomic mutation frequency of CTNNB1 (21.6% vs. 22.6%), AXIN1 (14.4% vs. 13.7%), RB1 (11.7% vs. 11.9%) in our cohort was not significantly different from previous reports [28]. However, the mutation frequency of TP53 (50.5% vs. 56.5%) and TERT (34% vs. 45.2%) in our cohort are lower to the cohort of Wang et al. [28]. We deduced that the difference in the number of patients with HBV infection may be one of the reasons. Some studies have reported that the mutation frequency of TP53 was higher in HCC caused by HBV infection than those without HBV infection [28, 29]. In the cohort of Wang et al., 84.8% (140/165) of patients were positive for hepatitis B surface antigen (HBsAg), while in our cohort, only 54.1% (60/111) of patients were positive for HBsAg. Moreover, Wang et al. counted more mutation types than us, including gene amplification and fusion/rearrangement. Another reason might be that the criteria for patient enrollment are different. We screened HCC patients with somatic mutations and excluded patients without somatic mutations detected by targeted-capture sequencing. This might lead to a change in the frequency of gene mutations.
The immunological analysis revealed that mutations in TP53 were related to the level of immune infiltration. A report showed that HCC patients with mutant TP53 had significantly macrophage infiltration higher than those with wild-type TP53 [30], which coincided with our results. Loss or alteration of p53 caused by TP53 mutations can regulate the recruitment and activation of immune cells, resulting in the suppression or evasion of anti-tumor immune responses [31, 32]. TP53 mutants can reprogram macrophages to tumour-associated macrophages (TAMs) [33] and were found to relate to the infiltration of TAMs into primary tumours [34]. One possible mechanism is that TP53 mutants lead to increased expression of the chemokine CCL2. CCL2, through the CCL2-CCR2 signalling axis, recruits TAMs to the tumour area [34, 35]. Given the profound impact of the TP53 status of the cancer cell on the immune response, previous studies have found that TP53 mutations have the potential to serve as a predictive factor in guiding anti-PD-1/PD-L1 immunotherapy [36, 37]. Because TP53 mutation significantly increased the expression of PD-1 and PD-L1. These studies focus on lung adenocarcinoma. High expression of PD-1 or PD-L1 has been consistently identified as a reliable predictor of a positive response to immunotherapy in various types of cancer. However, the association between TP53 and PD-L1 expression varies among cancer types [38, 39]. Indeed, no definitive biomarkers have been identified to predict the efficacy of immunotherapy in HCC. Studies on PD-L1 expression in HCC are limited or have limited clinical value due to their low occurrence frequencies. The positive rate of PD-L1 expression in HCC tumour cells ranges from 10 to 20% [40], but objective responses have been observed in PD-1 monotherapy regardless of PD-L1 expression [41, 42]. Therefore, more comprehensive and in-depth research is needed to determine whether TP53 mutants can serve as biomarkers for immune therapy in HCC.
Approximately 25% of potentially actionable mutations are found in HCC [6]. Unfortunately, the most prevalent drivers and trunk mutations, such as TERT promoter, AXIN1, and TP53 mutations, are currently undruggable [43]. Nevertheless, recent studies have shown that there are already relevant, targeted drugs in Phase I to III trials [44]. For instance, CTNNB1 mutation-blocking drugs are expected to be useful for precision medicine [44]. A Japanese early clinical experience explored the effect of Atezolizumab plus Bevacizumab (ATZ/BV) in HCC patients harbouring CTNNB1 Mutation and found that ATZ/BV might improve the immunosuppressive tumour microenvironment caused by CTNNB1 mutation [45]. Patients harbouring CTNNB1 mutations are mainly manifested as immune rejection in the previous study [46] and our study. Thus, 25 patients harboring CTNNB1 mutations in our study might use ATZ/BV to improve immunosuppression. Additionally, Lim et al. conducted a phase II clinical study on treating RAS-mutant HCC using refametinib or refametinib plus sorafenib, which has shown promise [47]. This provided new potential treatment options for RAS-mutant HCC patients.
Notably, we found that mutant MUC16 was an independent protective factor for the prognosis of HCC patients after curative hepatectomy. MUC16, encoding CA125, is the second most commonly mutated gene in HCC and has the most novel potential pathogenic variants in our study. Mutant MUC16 was also found to result in a better prognosis in gastric cancer and low-grade glioma [48,49,50]. The mechanisms underlying the favourable prognosis associated with MUC16 mutations remain unclear. In gastric cancer research, the group with MUC16 mutations showed increased infiltration of tumour-killing cells and decreased presence of immunosuppressive cells [48]. The infiltration of immune cells may significantly contribute to a better prognosis. However, we did not observe any differences in immune cell infiltration between the MUC16 mutation group and the wild-type group in our study on HCC. The mechanisms by which MUC16 mutation leads to a better prognosis may vary across different tumours. Therefore, MUC16 mutations may assist in HCC prognosis and should be further studied in this tumour type. Moreover, we observed that positive HBsAg was a risk factor for prognosis in multivariable analysis, but HBsAg did not show a significant association with prognosis in univariate analysis. A possible reason is that the effects of other factors are eliminated through multivariable analysis, revealing the independent effect of HBsAg on prognosis.
There are several limitations to our study. Firstly, targeted sequencing cannot detect changes in genes excluded from the assay, structure variation, and HBV/HCV integration. Secondly, sampling a single site cannot represent the whole tumour since HCC is highly heterogeneous. Thirdly, this study should be continued to collect more information on postoperative treatment and patient survival to link drug response and prognosis with molecular profiles. Fourthly, the average follow-up time of this study was not long enough (slightly over one year) to assess persistence of the impact of mutations. Despite these limitations, we identified novel potential immunotherapy efficacy and prognosis predictors.
Linking genomic alterations to clinical practice can identify patients who are likely to benefit from targeted therapies or immunotherapy and have a poor prognosis. We hope that our findings will make routine genetic testing more accessible in clinical practice and a research context.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is(are) available in the China National Center for Bioinformation (CNCB) repository, [unique persistent identifier and hyperlink to dataset(s) in https://ngdc.cncb.ac.cn/gvm/getProjectDetail?project=GVM000754].
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- OS:
-
Overall survival
- SNV:
-
Single nucleotide variants
- IQR:
-
Interquartile range
- HR:
-
Hazard ratio
- ATZ/BV:
-
Atezolizumab plus Bevacizumab
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19(2):223–38.
Marengo A, Rosso C, Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016;67:103–17.
Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM: Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina (Kaunas, Lithuania) 2019, 55(9):526.
Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C, Zucman-Rossi J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218.
Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47(5):505–11.
Fujimoto A, Furuta M, Shiraishi Y, Gotoh K, Kawakami Y, Arihiro K, Nakamura T, Ueno M, Ariizumi S, Nguyen HH, et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun. 2015;6:6120.
Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44(6):694–8.
de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95(15):8847–51.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Sorafenib. Profiles Drug Subst Excip Relat Methodol. 2019;44:239–66.
Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105(9):1087–92.
Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239.e1224.
Li L, Rao X, Wen Z, Ding X, Wang X, Xu W, Meng C, Yi Y, Guan Y, Chen Y, et al. Implications of driver genes associated with a high tumor mutation burden identified using next-generation sequencing on immunotherapy in hepatocellular carcinoma. Oncol Lett. 2020;19(4):2739–48.
Woo HG, Wang XW, Budhu A, Kim YH, Kwon SM, Tang ZY, Sun Z, Harris CC, Thorgeirsson SS. Association of TP53 mutations with stem cell-like gene expression and survival of patients with hepatocellular carcinoma. Gastroenterology. 2011;140(3):1063–70.
Liu F, Hou W, Liang J, Zhu L, Luo C. LRP1B mutation: a novel independent prognostic factor and a predictive tumor mutation burden in hepatocellular carcinoma. J Cancer. 2021;12(13):4039–48.
Song K, He F, Xin Y, Guan G, Huo J, Zhu Q, Fan N, Guo Y, Zang Y, Wu L. TSC2 mutations were associated with the early recurrence of patients with HCC underwent hepatectomy. Pharmgenomics Pers Med. 2021;14:269–78.
Chen Y, Chen Y, Shi C, Huang Z, Zhang Y, Li S, Li Y, Ye J, Yu C, Li Z, et al. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience. 2018;7(1):1–6.
Md V, Misra S, Li H, Aluru S. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS). 2019. p. 2314–24.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164.
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526(7571):68–74.
Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, Rudolph JE, Yaeger R, Soumerai T, Nissan MH, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017:PO.17.00011.
Gonzalez-Perez A, Perez-Llamas C, Deu-Pons J, Tamborero D, Schroeder MP, Jene-Sanz A, Santos A, Lopez-Bigas N. IntOGen-mutations identifies cancer drivers across tumor types. Nat Methods. 2013;10(11):1081–2.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Can Res. 2017;77(21):e108–10.
Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–56.
Jia W, Li H, Li S, Chen L, Li SC. Oviz-Bio: a web-based platform for interactive cancer genomics data visualization. Nucleic Acids Res. 2020;48(W1):W415–w426.
Wang S, Shi H, Liu T, Li M, Zhou S, Qiu X, Wang Z, Hu W, Guo W, Chen X, et al. Mutation profile and its correlation with clinicopathology in Chinese hepatocellular carcinoma patients. Hepatobiliary Surg Nutr. 2021;10(2):172–9.
Amaddeo G, Cao Q, Ladeiro Y, Imbeaud S, Nault JC, Jaoui D, Gaston Mathe Y, Laurent C, Laurent A, Bioulac-Sage P, et al. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut. 2015;64(5):820–9.
El-Arabey AA, Abdalla M, Abd-Allah AR. SnapShot: TP53 status and macrophages infiltration in TCGA-analyzed tumors. Int Immunopharmacol. 2020;86:106758.
Carlsen L, Zhang S, Tian X, De La Cruz A, George A, Arnoff TE, El-Deiry WS. The role of p53 in anti-tumor immunity and response to immunotherapy. Front Mol Biosci. 2023;10:1148389.
Blagih J, Buck MD, Vousden KH. p53, cancer and the immune response. J Cell Sci. 2020;133(5):jcs237453.
Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG, Harris CC. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9(1):771.
Walton J, Blagih J, Ennis D, Leung E, Dowson S, Farquharson M, Tookman LA, Orange C, Athineos D, Mason S, et al. CRISPR/Cas9-mediated Trp53 and Brca2 knockout to generate improved murine models of ovarian high-grade serous carcinoma. Can Res. 2016;76(20):6118–29.
Tesei A, Arienti C, Bossi G, Santi S, De Santis I, Bevilacqua A, Zanoni M, Pignatta S, Cortesi M, Zamagni A, et al. TP53 drives abscopal effect by secretion of senescence-associated molecular signals in non-small cell lung cancer. J Exp Clin Cancer Res. 2021;40(1):89.
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–24.
Biton J, Mansuet-Lupo A, Pécuchet N, Alifano M, Ouakrim H, Arrondeau J, Boudou-Rouquette P, Goldwasser F, Leroy K, Goc J, et al. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clin Cancer Res. 2018;24(22):5710–23.
Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y, Araujo L, Carbone DP, Shilo K, Giri DK, et al. PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst 2016;108(1):djv303.
Yadollahi P, Jeon YK, Ng WL, Choi I. Current understanding of cancer-intrinsic PD-L1: regulation of expression and its protumoral activity. BMB Rep. 2021;54(1):12–20.
Pinato DJ, Mauri FA, Spina P, Cain O, Siddique A, Goldin R, Victor S, Pizio C, Akarca AU, Boldorini RL, et al. Clinical implications of heterogeneity in PD-L1 immunohistochemical detection in hepatocellular carcinoma: the Blueprint-HCC study. Br J Cancer. 2019;120(11):1033–6.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim T-Y, Choo S-P, Trojan J, Welling TH 3rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52.
Llovet JM, Pinyol R, Kelley RK, El-Khoueiry A, Reeves HL, Wang XW, Gores GJ, Villanueva A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer. 2022;3(4):386–401.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616.
Ogawa K, Kanzaki H, Chiba T, Ao J, Qiang N, Ma Y, Zhang J, Yumita S, Ishino T, Unozawa H, et al. Effect of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma harboring CTNNB1 mutation in early clinical experience. J Cancer. 2022;13(8):2656–61.
Shimada S, Mogushi K, Akiyama Y, Furuyama T, Watanabe S, Ogura T, Ogawa K, Ono H, Mitsunori Y, Ban D, et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine. 2019;40:457–70.
Lim HY, Merle P, Weiss KH, Yau T, Ross P, Mazzaferro V, Blanc J-F, Ma YT, Yen CJ, Kocsis J, et al. Phase II Studies with Refametinib or Refametinib plus Sorafenib in Patients with RAS-Mutated Hepatocellular Carcinoma. Clin Cancer Res. 2018;24(19):4650–61.
Huang YJ, Cao ZF, Wang J, Yang J, Wei YJ, Tang YC, Cheng YX, Zhou J, Zhang ZX. Why MUC16 mutations lead to a better prognosis: a study based on the cancer genome atlas gastric cancer cohort. World J Clin Cases. 2021;9(17):4143–58.
Zhang F, Li X, Chen H, Guo J, Xiong Z, Yin S, Jin L, Chen X, Luo D, Tang H et al. Mutation of MUC16 is associated with tumor mutational burden and lymph node metastasis in patients with gastric cancer. Front Med (Lausanne). 2022;9:836892.
Ferrer VP. MUC16 mutation is associated with tumor grade, clinical features, and prognosis in glioma patients. Cancer Genet. 2023;270–271:22–30.
Funding
This work was supported by the grant of Peking University Shenzhen Hospital Foundation (Grant No.KYQD2022132).
Author information
Authors and Affiliations
Contributions
C.D.S and Y.N.W participated in the study conception and design. M.Q.S, H.Y.C and Z.R.T have performed analysis and interpretation of the data. M.Q.S, H.Z, K.M, L.F.L, Q.W and Z.J.X were involved in data analysis and interpretation. M.Q.S, H.Y.C, Z.R.T and Y.Z.Z prepared the manuscript and figures. H.Y.C conducted the statistical analysis. Y.N.W and C.D.S edited, critically read, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (approval no. QYFYWZLL27327). The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12885_2024_12407_MOESM1_ESM.docx
Additional file 1: Figure S1. Mutated genes in the TP53 pathway. Figure S2. The landscape of frequently mutated genes and chemotherapy-related genes in 111 patients. Figure S3. Association of immune infiltration with mutant genes.
12885_2024_12407_MOESM2_ESM.xlsx
Additional file 2: Table S1. Clinical information of 111 patients with Hepatocellular carcinoma. Table S2. List of the targeted genes. Table S3. Overview of the clinically actionable genomic alterations. Table S4. List of genes that have been detected by at least five software programs with new deleterious mutations. Table S5. The pathogenicity prediction results of the variants. Table S6. Thirteen mutation statuses stratified by clinical characteristics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, M., Cheng, H., Zou, H. et al. Genomic profiling informs therapies and prognosis for patients with hepatocellular carcinoma in clinical practice. BMC Cancer 24, 673 (2024). https://doi.org/10.1186/s12885-024-12407-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12407-2