- Research
- Open access
- Published:
Women’s country of birth and failure to catch up an overdue cervical cancer cytological screening participation during pregnancy in France, an observational study based on survey sources
BMC Cancer volume 24, Article number: 595 (2024)
Abstract
Background
Cervical cancer is the fourth most common cancer among women worldwide, both for incidence and mortality. Prevention relies on screening with a Pap test to detect precancerous lesions, which can then be treated. Access to this screening is currently both improvable and inequitable. Pregnancy may be an ideal moment for women to catch up on their overdue cervical cancer screening. In the general population, women's risk of not being screened is associated with their place of birth and other social factors; this may be true as well among pregnant women. Our objective was to study the association between women's place of birth and their failure to catch up with this screening during pregnancy.
Methods
The 2016 French National Perinatal Survey included 13,147 women who gave birth after 21 weeks of gestation. The association between their place of birth and failure to catch up on this screening (defined by the absence of a Pap test during pregnancy for women overdue for it) was adjusted for age, parity, education level, health insurance, and when they began prenatal care with logistic regression models.
Results
Among the women for whom screening was then recommended, 49% were not up to date at the start of pregnancy, and of these, 53% were not caught up before delivery. After adjustment for other risk factors, maternal place of birth was not associated with a higher risk of failure to catch up with this screening during pregnancy. However, factors identified as associated with this risk included a low education level and late start of prenatal care.
Conclusion
About half of women overdue for cervical cancer screening did not catch up with it during their pregnancy. Professionals should pay special attention to women with lower education levels and late initiation of prenatal care, who constitute a group at high risk of not catching up on this screening during pregnancy.
Background
The World Health Organization (WHO) reports that the incidence of cervical cancer was 660,000 worldwide in 2022 and that more than 350,000 women died from it that year [1].
Prevention relies on screening with a Pap test to detect precancerous lesions that can then be treated. In Europe, screening and treating early neoplasia have substantially reduced the incidence and mortality of cervical cancer since 1960 [2,3,4,5]. In this area in 2021, rates of women aged 30–49 who reported ever having had a cervical cancer test varied from 42.0% (in Romania) to 98.4% in Finland, according to the WHO [6].In other high-income countries, rates were higher: 88% in the USA, 91% in Canada, and 95% in Australia had ever undergo Pap tests [7].
From 2010 to 2019, French guidelines recommended that women have a Pap test every 3 years between the ages of 25 and 65 years, after they have had two normal Pap smear results one year apart [8]. These guidelines, however, have been poorly implemented in France: only 58.7% of women were screened every three years between 2015 and 2017 in France [9].
Low participation rates in screening programs increases the risk of dying from invasive cervical cancer, and every year in France, 60% to 70% of the new cases of this cancer are diagnosed among women aged from 35 to 69 years who are unscreened or underscreened [8]. Recent studies show that risk factors for such non-screening or underscreening include a low education level and/or low income, living alone, unemployment, and lack of medical insurance, compared with women living in more privileged environments [10,11,12,13,14,15,16,17,18,19,20,21]. Some studies have also found that migrant women are screened less often than native women [11, 22,23,24,25,26,27]. In Canada, migrant women have an adjusted RR 1.32; 95% CI 1.20–1.45) for an overdue Pap test compared to Canadian-born women [25]. Several French studies found that foreign women born to foreign parents underwent recommended cervical cancer screening less often than French women born to foreign parents, who themselves were less likely to be screened than French women born to French parents [12, 28, 29]. In Norway, Enden and al. showed that, despite a global increase of cervical cancer screening participation between 2012 et 2017, this increase was significantly smaller among immigrant women compared to Norwegian-born women [24].
Because pregnancy is a privileged moment for access to health care, it might be a good time to catch up with gynecologic follow-up for women not receiving regular triennial screening [8].
Although the performance of this screening has not been evaluated in pregnant women, the French Health Authority has recommended since 2007 Pap tests for all woman at the beginning of pregnancy if their last test took place more than three years earlier [30]. Maternal health inequalities according to maternal place of birth have been described in high-income countries, specifically in France [31, 32]. It is important to know if these inequalities also affect cervical cancer screening during pregnancy.
The objectives of this study were to describe the association between mothers' place of birth and their failure to catch up on cervical cancer screening during pregnancy and to identify whether some other social characteristics might be risk factors for this among a national sample of women giving birth in France.
Methods
Data sources
The study population came from the French National Perinatal Survey conducted in March 2016. These surveys are fairly regular population-based cross-sectional studies using the same methodology and including all births (live births and stillbirths) after 21 weeks’ gestation or with a birthweight of at least 500 g during a one-week period in all maternity units in France [33].
For each birth, data were collected by a face-to-face interview and the collection of information from the medical records by a midwife. Maternal socioeconomic characteristics and prenatal care were obtained during the interview. Each woman was asked about a Pap test during pregnancy and over the past three years.
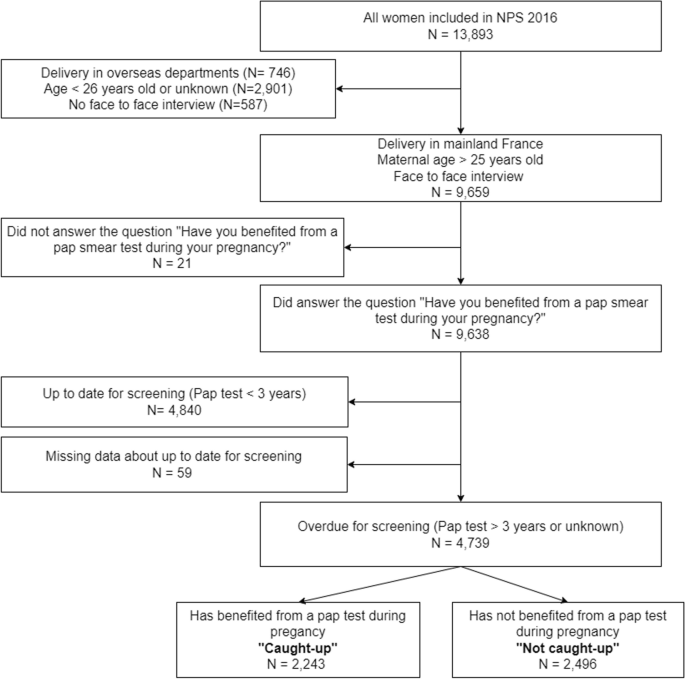
The perinatal survey database included 13,893 women (Fig. 1 Study population). The study population included all women who gave birth in mainland France, were more than 25 years old, and were interviewed and answered the question about a Pap test during pregnancy and over the past 3 years.
Variables
Outcome measurement
The outcome was the performance of a Pap test during pregnancy for women aged 26 years or older who were not up to date for this screening.
We considered women to be “up to date” for screening when they reported having had a Pap test in the previous three years. Women were considered overdue for it when they reported that their last Pap test took place more than three years earlier or that its date (if any) was unknown.
Among the overdue women, those who answered “Yes” to the question about a Pap test during pregnancy are classified as “caught up” and those who answered “No” or did not remember having had a Pap test during pregnancy were considered as “not caught up”.
Exposure measurement
Maternal place of birth was classified in five categories: France, other European countries, North Africa, other African countries, and elsewhere in the world.
Social and demographic characteristics
-
Maternal age was divided into three categories: 26–30 years old, 31–35 years old, or 36 years older or more.
-
Education level was the highest level of education, again in three categories: Middle school or less, high school and beyond high school.
-
Socioeconomic situation was defined by several characteristics:
-
Employment status during pregnancy: Employed, unemployed and/or looking for work, homemaker or student or other.
-
Personal housing during the last trimester of pregnancy, as a binary variable: yes or no.
-
No work-related household income, as a binary variable: yes or no.
-
Standard health insurance coverage at the beginning of pregnancy, as a binary variable: yes or no.
-
Living with a partner as a binary variable: yes or no.
Inadequate prenatal care utilization
We used the indicator described by Gonthier et al. [34] to assess the adherence of prenatal care to current French recommendations. It covers late initiation of care (started later than 12 weeks of gestation) and too few appointments (i.e. < 7 prenatal visits and 3 ultrasound examinations for full term pregnancies) and is defined specifically as:
-
-
Late initiation of care
And/or
-
-
Fewer than half the number of prenatal visits expected according to the duration of pregnancy
And/or
-
-
Insufficient number of ultrasound screenings: missing either the first-trimester ultrasound examination or both the second- and third-trimester examinations
Statistical analyses
The population was described by comparing the women overdue at the start of pregnancy who were and were not "caught up" by its end for proportions of categorical variables. To study the association between each social factors and failure to catch up on screening, we performed a bivariate analysis. Then, we constructed a multivariate logistic regression model adjusted for maternal place of birth, age, parity, education level, health insurance coverage, and timing of prenatal care initiation. Associations between failure to catch up, mother’s place of birth, and covariates were expressed as crude odds ratios (OR) and adjusted odds ratios (AOR) and their 95% confidence intervals (CIs).
STATA 15.0 software was used to perform the analyses.
Results
Description
Women included in the National Perinatal Survey and who gave birth in mainland France, were more than 25 years old (eligible for cervical cancer screening), and answered the question about a Pap test during pregnancy and over the past 3 years were 9,638 (Fig. 1 Study population). Among the latter, 4,840 women reported they were up to date for cervical cancer screening because they had had a Pap test in the previous three years, while 4,739 women (49%) were overdue. Among these overdue women, 2,243 (47%) answered the question about a Pap test during pregnancy positively and are considered caught up, while 1,862 (53%) answered negatively or did not remember and were considered not caught up.
Not caught up women were younger (chi-square p test = 0.001), less well educated (p < 0.0001), and less often employed (p = 0.002) than women who were caught up (Table 1). They also initiated prenatal care later than caught-up women and had an inadequate prenatal care utilization more frequently (p < 0.0001).
Factors associated with not catching up: bivariate and multivariate analyses
We did not observe with the bivariate analysis any association between maternal place of birth and failure to catch up during pregnancy (Table 2). The analysis however showed that several factors were associated with failing to catch up, including having non-standard (versus standard) health insurance at the beginning of pregnancy (Crude OR 1.34 95% CI [1.14–1.57]) and a middle school or high school education level (versus beyond high school level) (Crude OR 1.33 95% CI [1.15–1.53] and 1.27 95% CI [1.10–1.48] respectively).
In the multivariate analysis, after adjustment for age, parity, level of education, health insurance and late initiation of prenatal care, the maternal place of birth was not significantly associated with the risk of not being caught up. On the other hand, having a middle school or high school education level was significantly associated with not catching up (AOR 1.24 95% CI [1.06–1.45] and AOR 1.21 95% CI [1.04–1.41] respectively), compared with women with higher qualifications. Late initiation of antenatal care was strongly associated with failure to catch up (AOR = 2.13 95% CI [1.46–3.10]).
The proportion of missing data was less than 2% for each variable. Observations containing missing data were excluded from the multivariate analysis which was performed on 4,601 complete observations out of 4,739.
Discussion
This analysis of the 2016 French National Perinatal Survey shows that 49% of the women eligible for cervical screening were overdue for it, and among this group, 53% did not catch up with this screening during their pregnancy, despite national guidelines strongly recommending it. Maternal place of birth was not associated with this failure to catch up during pregnancy, although an age of 26–30 years, a lower education level, a start of prenatal care later, compared with overdue women who were caught up, were associated with it.
Strengths and limitations
One of the strengths of this analysis is the large number of women included and the low rate of missing data; these factors together provide good statistical power and limit the risk of bias. The survey's design also ensures the sample's representativity. The participation of nearly every maternity unit in France resulted in a number of births very close to that expected according to the INSEE statistics; at the same time, the characteristics of the mothers, deliveries, and newborns were similar to those already known through hospital discharge summaries (PMSI) [33].
Nonetheless, women not speaking French well did not have face-to-face interviews and were thus excluded from this analysis. They accounted for almost 4% of the women aged 26 years or older. Most of them were immigrants and perhaps among the most deprived individuals in our sample. This selection bias might have led us to underestimate the strength of the associations between social factors and failure to catch up. On the other hand, excluding these women from the study and analysis might have prevented us from being able to highlight an existing association between immigration and catch-up failure.
Another limitation is related to the quality of the data collected about prenatal care. Women may have forgotten, omitted, or misunderstood some questions. They may confuse Pap tests with simple vaginal samples. A few studies suggested that women over report Pap tests, partly by equating any examination of the pelvic area to a Pap test [9, 35,36,37]. Women with a low level of education or with a language barrier may therefore have more often misunderstood this question; some women may not have been considered caught up although they had had a Pap test, or the inverse might be true.
While many authors have asked if social and economic status influences the rate of reporting the response is not unanimous: some authors find over-reporting among the most disadvantaged, others among the most advantaged, while still others find no association between social background and reporting [35, 36, 38]. Lastly, during the National Perinatal Survey, the interview was carried out by a midwife, who could help women remember this test and could have limited memorization bias.
Interpretation of results
Failure to catch up
First, almost half of all pregnant women were overdue for cervical cancer screening in France in 2016, and slightly more than half did not catch up during pregnancy. French hospital-based studies have found similar rates of failure to catch up during pregnancy (from 53 to 61%) [39,40,41], but our work is the first study to describe this phenomenon among a national sample of pregnant women. In the UK, Coleridge et al. found that nearly half (47.3%) of a sample of 260 pregnant women were overdue for cervical screening and 74% were not caught up during either their pregnancy or the first 6 months postpartum [42]. In Brazil, Terlan and Cesar have observed that, despite prenatal visits, 21.6% pregnant women did not undergo the Pap smears they should have had [43].
Despite these inadequate catch-up rates during pregnancy, some countries have shown that this period does indeed present an important opportunity for health care professionals to help women to catch up with overdue screening. A Norwegian cohort study including more than 2 million women showed that pregnant women were almost five times more likely to have a Pap smear test within one year compared to the non-pregnant women [44]. A Polish hospital-based study found that 7.5% of women older than 25 years reported that the Pap test performed during pregnancy, in accordance with local guidelines, was the first they had ever had.
Maternal place of birth
In our analysis, maternal place of birth was not associated with failure to catch up with cervical cancer screening during pregnancy. To our knowledge, this study is the first to assess specifically the association between maternal place of birth and this screening during pregnancy. Moreover, we have not found studies that investigated the associations between maternal nationality or ethnicity and cervical cancer screening. Most studies concern associations between women’s place of birth or ethnicity in general populations.
Several Canadian studies have shown significant cervical cancer screening inequalities based on age, income, immigration status, and world region of origin [25, 27]. A review of the literature conducted in 2019 showed that women from sub-Saharan Africa and living in Canada origin had the lowest cervical cancer screening rates [45].
In Norway, women from North and sub-Saharan Africa had lower rates of participation in cervical cancer screening programs than Norwegian-born women (adjusted OR 0.61, 95% CI [0.56–0.67]) [46]. In Denmark, migrant women have the lowest rate of participation in the national screening program, even after adjustment for other social characteristics. The authors suggest that this result might be due to a language barrier, some difficulties in understanding the screening invitation (written in Danish), and poor health literacy — all barriers to seeking care or understanding and adhering to prevention and screening [11, 47]. According to Idehen et al., Russians, Somalis and Kurds women living in Finland are less screened than Finnish women [26].
In France, Sassenou et al. observed in 2023 that women residing in France and born in European countries other than France were screened less often than native women [29]. The lack of association between maternal place of birth and catch-up screening during pregnancy, analyzed in a selected population of overdue pregnant women, does not however reflect an association that would exist outside pregnancy between place of birth and access to cervical cancer screening.
Age
Age was also associated with failure to catch up. In our study, the youngest pregnant women had had fewer Pap tests than those older than 30 years. In the Polish study by Kusczborska et al., age was the only factor associated with Pap tests both before and during the current pregnancy, but it enrolled women younger than 25 years, who are normally not subject to Polish screening guidelines [48]. In Brazil, Monteiro et al. and Cesar et al. found that young age (younger than 35 years old, respectively) was associated with lower Pap testing rates during pregnancy [49, 50].
Adherence to medical guidelines
Late initiation of prenatal care was associated with failure to catch up on screening. This may be due to the care provider's concern about performing a Pap test after the first trimester and suggests poor knowledge of current guidelines. The French Health Authority guidelines, the French Public Health Code, and the guidelines of the French National College of Gynecologists and Obstetricians state that a Pap test can be performed at any time during pregnancy, especially for women without regular gynecological follow-up [40, 51]. Nonetheless, among a sample of French midwives interviewed in 2018, 29% reported that they would perform a Pap test at 25 weeks of gestation, compared with more than 90% at 10 weeks [52]. In a study that took place in 2009–2010 in a University Hospital Center of France, the proportion of adequate screening (defined by performing a Pap test during pregnancy if the last one was more than two years earlier or if its result was unknown) was significantly higher when the first prenatal visit occurred during the first trimester rather than during the second or the third trimester (48% versus 12%) [41]. According to Saulneron et al. most Pap tests performed during pregnancy take place during the first trimester (86.7%) [40]. In the Norwegian cohort of Nygard et al., most Pap smears from pregnant women were taken during the first 4 months of pregnancy [44].
A Pap test can also be proposed during the postnatal visit, but several studies have shown that 68% to 83% of women do not attend this visit, in particular, those in situations of social deprivation [53,54,55]. This non-adherence results in missing the opportunity for these women to be caught up with this important preventive care, in particular, those with a poor access to gynecological care [56, 57].
Strong public health policies could reduce the late initiation of prenatal care and thereby have a positive impact on cervical cancer screening during pregnancy. In Norway, the high rate of participation of pregnant women in the national screening program has improved its coverage throughout the female population [44].
In 2019, the French Health Authority (HAS) published new recommendations on cervical cancer screening, advising an HPV test every five years for women over 30, rather than Pap tests [58]. These new guidelines, if well disseminated to and adhered to by health care providers, may improve screening of pregnant woman overdue for cervical cancer screening.
Individual factors play a moderate role in failed catch up of women overdue for screening during pregnancy. A better understanding of why recommendations are so poorly implemented requires a study of the knowledge, attitudes, and practices of all health care providers.
Conclusion
Despite guidelines, nearly half of all pregnant women are overdue for cervical cancer screening, and catch-up will not occur for 53% of them during pregnancy. A young age (younger than 30 years), a low education level, and late initiation of prenatal care are factors associated with failure to catch up, but maternal place of birth does not appear to be an independent risk factor. Health care professionals must be made aware of these factors, so that women who are overdue for screening, particularly those most at risk, can catch up. It is important that professionals involved in prenatal care understand the new screening procedures well and can implement them, even for women whose prenatal care begin late.
Availability of data and materials
A description of the study is available from: enp.inserm.fr. The data are partially accessible from the following link http://quetelet.progedo.fr/.
References
WHO. Cervical cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
Defossez G, Le Guyader-Peyrou S, Uhry Z, Grosclaude P, Colonna M, Dantonny E, et al. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018 - Tumeurs solides : Étude à partir des registres des cancers du réseau Francim. Available from: https://www.santepubliquefrance.fr/import/estimations-nationales-de-l-incidence-et-de-la-mortalite-par-cancer-en-france-metropolitaine-entre-1990-et-2018-tumeurs-solides-etude-a-partir. Cited 2024 Apr 2.
Lapôtre-Ledoux B, Remontet L, Uhry Z, Dantonny E, Grosclaude P, Molinié F, et al. Incidence des principaux Cancers en France Métropolitaine en 2023 et tendances depuis 1990. Bull Épidémiol Hebd. 2023;12–13:188–204.
Jansen EEL, Zielonke N, Gini A, Anttila A, Segnan N, Vokó Z, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer. 2020;127:207–23.
Pedersen K, Fogelberg S, Thamsborg LH, Clements M, Nygård M, Kristiansen IS, et al. An overview of cervical cancer epidemiology and prevention in Scandinavia. Acta Obstet Gynecol Scand. 2018;97(7):795–807.
Williams J, Rakovac I, Victoria J, Tatarinova T, Corbex M, Barr B, et al. Cervical cancer testing among women aged 30–49 years in the WHO European Region. Eur J Pub Health. 2021;31(4):884–9.
WHO. Complete cervical cancer profile sets. 2021. Available from: https://www.who.int/publications/m/item/cervical-cancer-country-profiles. Cited 2024 Apr 11
État des lieux et recommandations pour le dépistage du cancer du col de l’utérus en France. HAS; 2010. Available from: https://www.has-sante.fr/jcms/c_1009772/fr/etat-des-lieux-et-recommandations-pour-le-depistage-du-cancer-du-col-de-l-uterus-en-france.
Hamers F, Jezeweski-Serra D. Couverture du dépistage du cancer du col de l’utérus en France, 2012–2017. Revue de Biologie Médicale/N. 2020;353:67–74.
Levinson KL, Jernigan AM, Flocke SA, Tergas AI, Gunderson CC, Huh WK, et al. Intimate partner violence and barriers to cervical cancer screening: a gynecologic oncology fellow research network study. J Low Genit Tract Dis. 2016;20(1):47–51.
Harder E, Juul KE, Jensen SM, Thomsen LT, Frederiksen K, Kjaer SK. Factors associated with non-participation in cervical cancer screening–a nationwide study of nearly half a million women in Denmark. Prev Med. 2018;111:94–100.
Grillo F, Vallée J, Chauvin P. Inequalities in cervical cancer screening for women with or without a regular consulting in primary care for gynaecological health, in Paris, France. Prevent Med. 2012;54(3–4):259–65.
Konopka AM, Barnay T, Billaudeau N, Sevilla-Dedieu C. Les déterminants du recours au dépistage du cancer du col de l’utérus: une analyse départementale. Econ Prév. 2019;2:43–63.
Ricardo-Rodrigues I, Jiménez-García R, Hernández-Barrera V, Carrasco-Garrido P, Jiménez-Trujillo I, de Andrés AL. Social disparities in access to breast and cervical cancer screening by women living in Spain. Public Health. 2015;129(7):881–8.
Petkeviciene J, Ivanauskiene R, Klumbiene J. Sociodemographic and lifestyle determinants of non-attendance for cervical cancer screening in Lithuania, 2006–2014. Public Health. 2018;156:79–86.
Guthmann JP, Pelat C, Parent du Chatelet I, Duport N, Lévy-Bruhl D, Institut de Veille Sanitaire (InVS). Déterminants socio-économiques de vaccination et de dépistage du cancer du col par frottis cervico-utérin (FCU). Analyse de l’Enquête santé et protection sociale (ESPS), 2012. 2014. Available from: https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/documents/rapport-synthese/determinants-socio-economiques-de-vaccination-et-de-depistage-du-cancer-du-col-par-frottis-cervico-uterin-fcu-.-analyse-de-l-enquete-sante-et-prot.
Barré S, Massetti M, Leleu H, Catajar N, De Bels F. Caractérisation des femmes ne réalisant pas de dépistage du cancer du col de l’utérus par frottis cervico-utérin en France. Bull Epidémiol Hebd. 2017;2–3:39.
Sassenou J, Ringa V, Zins M, Ozguler A, Paquet S, Panjo H, et al. Women with obesity in cervical cancer screening. The double penalty: Underscreening and income inequalities. Obes Res Clin Pract. 2021;15(3):212–5.
Le Bihan-Benjamin C, Marchadier A, Audiger C, Khati I, Barré-Pierrel S. Quel déploiement du Programme national de dépistage organisé du cancer du col de l’utérus en France en 2022 ? Bull Épidémiol Hebd. 2024;5:82–91.
Bryere J, Dejardin O, Launay L, Grosclaude P, Launoy G, Réseau Français des regristres de cancers Francim. Environnement socioéconomique et incidence des cancers en France. Bull Épidémiol Hebd. 2017;4:68–77.
Traoré M, Vallée J, Chauvin P. Risk of late cervical cancer screening in the Paris region according to social deprivation and medical densities in daily visited neighborhoods. Int J Health Geogr. 2020;19(1):18.
Kristensson JH, Sander BB, von Euler-Chelpin M, Lynge E. Predictors of non-participation in cervical screening in Denmark. Cancer Epidemiol. 2014;38(2):174–80.
Rondet C, Lapostolle A, Soler M, Grillo F, Parizot I, Chauvin P. Are immigrants and nationals born to immigrants at higher risk for delayed or no lifetime breast and cervical cancer screening? The results from a population-based survey in Paris metropolitan area in 2010. PLoS ONE. 2014;9(1):e87046.
Enden MR, Møen K, Igland J, Diaz E. Trends in cervical cancer screening in Norway 2012–2017: a comparison study of non-immigrant and immigrant women. Scand J Public Health. 2024J;2:14034948231217636.
Bacal V, Blinder H, Momoli F, Wu KY, McFaul S. Is immigrant status associated with cervical cancer screening among women in Canada? Results from a cross-sectional study. J Obstet Gynaecol Can. 2019;41(6):824-831.e1.
Idehen EE, Koponen P, Härkänen T, Kangasniemi M, Pietilä AM, Korhonen T. Disparities in cervical screening participation: a comparison of Russian, Somali and Kurdish immigrants with the general finnish population. Int J Equity Health. 2018;17(1):56.
Vahabi M, Lofters AK, Kopp A, Glazier RH. Correlates of non-adherence to breast, cervical, and colorectal cancer screening among screen-eligible women: a population-based cohort study in Ontario, Canada. Cancer Causes Control. 2021F;32(2):147–55.
Crampe-Casnabet C, Franck JE, Ringa V, Coeuret-Pellicer M, Chauvin P, Menvielle G. Role of obesity in differences in cervical cancer screening rates by migration history. The Constances survey. Cancer Epidemiol. 2019;58:98–103.
Sassenou J, Ringa V, Zins M, Ozguler A, Paquet S, Panjo H, et al. Combined influence of immigration status and income on cervical cancer screening uptake. Prev Med Rep. 2023;36:102363.
Suivi et orientation des femmes enceintes en fonction des situations à risque identifiées. HAS; 2007. Available from: http://www.cfef.org/archives/bricabrac/suivigrossesse.pdf.
Eslier M, Azria E, Chatzistergiou K, Stewart Z, Dechartres A, Deneux-Tharaux C. Association between migration and severe maternal outcomes in high-income countries: Systematic review and meta-analysis. PLoS Med. 2023;20(6):e1004257.
Eslier M, Deneux-Tharaux C, Sauvegrain P, Schmitz T, Luton D, Mandelbrot L, et al. Severe maternal morbidity among undocumented migrant women in the PreCARE prospective cohort study. BJOG. 2022;129(10):1762–71.
Blondel B, Coulm B, Bonnet C, Goffinet F, Le Ray C. National coordination group of the national perinatal surveys. Trends in perinatal health in metropolitan France from 1995 to 2016: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46(10):701–13.
Gonthier C, Estellat C, Deneux-Tharaux C, Blondel B, Alfaiate T, Schmitz T, et al. Association between maternal social deprivation and prenatal care utilization: the PreCARE cohort study. BMC Pregnancy Childbirth. 2017;17:126.
Aranda E, Franck JE, Ringa V, Sassenou J, Coeuret-Pellicer M, Rigal L, et al. Social inequalities in participation in cancer screening: does the mode of data collection matter? The CONSTANCES cohort. European Journal of Public Health. 2021;31(3):602–8.
Lofters A, Vahabi M, Glazier RH. The validity of self-reported cancer screening history and the role of social disadvantage in Ontario, Canada. BMC Public Health. 2015;15(1):28.
Anderson J, Bourne D, Peterson K, Mackey K. Evidence brief: accuracy of self-report for cervical and breast cancer screening. Washington (DC): Department of Veterans Affairs (US). 2019.
Lofters AK, Moineddin R, Hwang SW, Glazier RH. Does social disadvantage affect the validity of self-report for cervical cancer screening? Int J Womens Health. 2013;5:29–33.
Moussier M. Réalisation du frottis cervico-utérin de rattrapage pendant la grossesse : profil des patientes concernées et informations reçues à propos du dépistage du cancer du col de l’utérus : étude observationnelle multicentrique dans le département des Alpes-Maritimes. 2019. Available from: https://dumas.ccsd.cnrs.fr/dumas-02271416/document. Cited 2020 Jul 22.
Saulneron M, Descamps-Mollier M, Carcopino X. Analyse de la pratique du frottis cervico-utérin de dépistage pendant la grossesse en France : étude bicentrique rétrospective de cohorte. J Gynecol Obstet Biol Reprod. 2015;44(6):516–23.
Gauchotte E. Frottis cervico-vaginaux pendant la grossesse : évaluation des pratiques professsionnelles et suivi des anomalies. 2011.
Coleridge SL, Wiggans A, Nelissen E, Bethune R, Blackwell R, Bryant A, et al. Improving the uptake of cervical screening in pregnant and recently postnatal women: a quality improvement project. BMJ Open Qual. 2022;11(2):e001709.
Terlan RJ, Cesar JA. Non-performance of Pap smears among pregnant women in the Extreme South of Brazil: prevalence and associated factors. Cien Saude Colet. 2018;23(11):3557–66.
Nygård M, Daltveit AK, Thoresen SØ, Nygård JF. Effect of an antepartum Pap smear on the coverage of a cervical cancer screening programme: a population-based prospective study. BMC Health Serv Res. 2007;7(1):10.
Nnorom O, Findlay N, Lee-Foon NK, Jain AA, Ziegler CP, Scott FE, et al. Dying to learn: a scoping review of breast and cervical cancer studies focusing on black Canadian women. J Health Care Poor Underserved. 2019;30(4):1331–59.
Møen KA, Kumar B, Qureshi S, Diaz E. Differences in cervical cancer screening between immigrants and nonimmigrants in Norway: a primary healthcare register-based study. Eur J Cancer Prev. 2017;26(6):521–7.
Ruel J, Moreau AC, Ndengeyingoma A, Arwidson P, Allaire C. Littératie en santé et prévention du cancer. Sante Publique. 2019;S2(HS2):75–8.
Kuczborska K, Kacperczyk-Bartnik J, Wolska M, Pluta M, Bartnik P, Dobrowolska-Redo A, et al. Secondary cervical cancer prevention in routine prenatal care — coverage, results and lessons for the future. Ginekol Pol. 2019;90(7):396–402.
Monteiro PB, Monteiro Filho MP, De Figueiredo JT, Saintrain MVDL, Bruno ZV, Carvalho FHC. Cytology-based screening during antenatal care as a method for preventing cervical cancer. Asian Pac J Cancer Prev. 2017S 1;18(9):2513–8.
Cesar JA, dos Santos GB, Sutil AT, Cunha CF, Dumith SD. [Pap smears among pregnant women in Southern Brazil: a representative cross-sectional survey]. Rev Bras Ginecol Obstet. 2012;34(11):518–23.
Recommandations pour la pratique clinique : Prévention du cancer du col de l’utérus. Collège national des gynécologues et obstétriciens français; 2007 Dec p. 391–404. Available from: http://www.cngof.fr/pratiques-cliniques/recommandations-pour-la-pratique-clinique/apercu?path=RPC%2BCOLLEGE%252F2007%252Frpc_prev-K-col2007.pdf&i=21959Cited 2020 Jul 22.
Kervella L, Berveiller P, Bourdillon M, Rousseau A. Midwives’ practices related to cervical cancer screening during pregnancy: A vignette-based study. Sex Reprod Healthc. 2020;26:100539.
Puech M. La consultation du post-partum: une consultation négligée? [PhD Thesis]. France: Université Toulouse III-Paul Sabatier; 2019.
Coget C. Connaissances, pratiques et attentes des femmes concernant la visite post-natale. Étude quantitative [PhD Thesis]. Faculté Mixte de Médecine et de Pharmacie de Rouen; 2018. Disponible sur: https://dumas.ccsd.cnrs.fr/dumas-02047837.
Polk S, Edwardson J, Lawson S, Valenzuela D, Hobbins E, Prichett L, et al. Bridging the postpartum gap: a randomized controlled trial to improve postpartum visit attendance among low-income women with limited English Proficiency. Womens Health Rep (New Rochelle). 2021;2(1):381–8.
Généralisation du dépistage du cancer du col de l’utérus /Étude médico-économique /Phase 1,appui à la décision [Internet]. INCA; 2015. Available from: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Generalisation-du-depistage-du-cancer-du-col-de-l-uterus-etude-medico-economique-Phase-1
Brun-Micaleff E, Coffy A, Rey V, Didelot MN, Combecal J, Doutre S, et al. Cervical cancer screening by cytology and human papillomavirus testing during pregnancy in French women with poor adhesion to regular cervical screening. J Med Virol. 2014;86(3):536–45.
HAS. Évaluation de la recherche des papillomavirus humains (HPV) en dépistage primaire des lésions précancéreuses et cancéreuses du col de l’utérus et de la place du double immuno-marquage p16/Ki67. 2019. Available from: https://www.has-sante.fr/jcms/c_2806160/fr/evaluation-de-la-recherche-des-papillomavirus-humains-hpv-en-depistage-primaire-des-lesions-precancereuses-et-cancereuses-du-col-de-l-uterus-et-de-la-place-du-double-immuno-marquage-p16/ki67. Cited 2020 Jun 8.
Acknowledgements
The authors thank the department head in each maternity unit who agreed to have the survey performed in their unit, the investigators and all the women who agreed to participate. EL would particularly like to thank Dr. J. Merrer, Midwife, PhD for her assistance with the data analysis and writing of the article.
Funding
The 2016 National Perinatal Survey was developed and implemented by the French National Institute of Health and Medical Research (INSERM), three directorates of the Ministry of Social Affairs and Health and the French National Public Health Agency (Santé Publique France).
Author information
Authors and Affiliations
Contributions
BB contributed to the conception of the National Perinatal Survey and was responsible for the data collection and dissemination of the main results. EL, SV, and EA performed the analyses and drafted the manuscript. SW critically reviewed the manuscript and contributed to substantial improvements. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The 2016 French National Perinatal Survey was approved by the French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés, approval no.: 915197), the National Council on Statistical Information (Comité du Label, approval no.: 2016X703SA), and the French Institute of Health and Medical Research Ethics Committee (INSERM Ethics Committee approval no.: IRB00003888 no. 14–191). Oral informed consent was obtained from women for survey participation before the interview.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lyonnais, E., Vigoureux, S., Blondel, B. et al. Women’s country of birth and failure to catch up an overdue cervical cancer cytological screening participation during pregnancy in France, an observational study based on survey sources. BMC Cancer 24, 595 (2024). https://doi.org/10.1186/s12885-024-12335-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12335-1