- Research
- Open access
- Published:
Experiences and perceptions of men following breast cancer diagnosis: a mixed method systematic review
BMC Cancer volume 24, Article number: 179 (2024)
Abstract
Background
Men with breast cancer experience unique physical and emotional challenges. However, a thorough understanding of these experiences including the psychosocial effects and supportive care needs have received less attention. In some settings, men with breast cancer experience stigma within the healthcare system and their care needs are not prioritised. This influences the level of professional support offered, consequently worsening their health and well-being outcomes. This review explored the variabilities in the experiences and treatment modalities of male breast cancer (MBC) across different contexts.
Methods
All primary study designs including qualitative, quantitative, and mixed methods studies that reported on the experiences, treatment approaches and outcomes of MBC were included in this systematic review. Six databases (Embase, Medline, PsycINFO, Global Health, CINAHL and Web of Science) were searched for articles from January 2000 to September 2023. A results-based convergence synthesis was used for data analysis and reported using PRISMA guidelines.
Results
Of the studies screened (n = 29,687), forty-four fulfilled the predetermined criteria and were included. Our findings relating to the experiences and treatment approaches of MBC are broadly themed into three parts. Theme 1—Navigating through a threat to masculinity: describes how males experienced the illness reflecting on detection, diagnosis, coming to terms with breast cancer, and disclosure. Theme 2- Navigating through treatment: captures the experiences of undergoing breast cancer treatment/ management following their diagnosis. Theme 3—Coping and support systems: describes how MBC patients coped with the disease, treatment process, aftercare/rehabilitative care, and the available support structures.
Conclusions
Men experience a myriad of issues following a breast cancer diagnosis, especially with their masculinity. Awareness creation efforts of MBC among the public and healthcare practitioners are urgently required, which could change the perception of men in promoting early diagnosis, adherence to treatments, post-treatment monitoring, oncological results and a better quality of life. Considerations for training, education and development of specialised guidelines for healthcare practitioners on MBC would provide the necessary knowledge and skills to enhance their practice through the adoption of person-centred and male-specific care strategies. Professional care intervention and support for MBC should not end after the diagnosis phase but should extend to the entire treatment continuum and aftercare including future research focusing on MBC specific clinical trials.
Trial registration
PROSPERO Registration No. CRD42021228778.
Background
Male breast cancer (MBC) is a rare condition, accounting for less than 1% of all breast cancers. About 2,710 men are estimated to be diagnosed with breast cancer, with approximately 530 men projected to die from breast cancer in 2022 and have about 1 in 833 lifetime risk of being diagnosed with the disease in the United States [1]. Data from the Global Burden of Disease 2017 database indicate that the incidence of MBC increased from 8.5 thousand in 1990 to 23.1 thousand in 2017 with 123 countries showing a significant increasing trend in MBC incidence rates [2]. There are variations in the incidence of MBC among countries for instance, in Thailand MBC incidence was lower than that in Israel, and the rate of variability has been attributed to population-specific factors [3]. Additionally, disparities have been noted in the incidence, prevalence, mortality, and burden of cancer and related adverse health conditions in specific population groups [4]. Some of these disparities have been noted in the United States, where black men are reported to have higher incidence and mortality rates compared to white men in the context of all cancer [4,5,6].
Evidence suggests that MBC is mostly diagnosed late (49%) when the disease is more advanced compared to women (33%) leading to relatively worse prognosis [7,8,9,10,11]. This has been attributed to delayed presentation, lack of screening, reduced awareness by treating providers and a lack of awareness of the disease among men [12,13,14,15]. Consequently, MBCs are mainly diagnosed with more severe clinical manifestations with relatively complex tumour characteristics (i.e., larger sizes and extensive lymph node involvement) [16], associated with higher proportions of positive hormone receptors, which mostly results in prolonged treatment delay, and metastasis of the disease at diagnosis compared to female breast cancer [17]. This has been influenced by issues with lower socioeconomic status, barriers to accessing healthcare and insurance cover issues in the context of the United States, adherence to treatment, post-treatment follow-up, and stigma [7, 18,19,20]. MBC patients suffer from a triple stigma including stigma by healthcare professionals, society, and especially by themselves as they struggle to accept the disease which has been labelled as a woman's disease [20].
Treatment for MBC has mainly been informed by available evidence for female breast cancer [21], and no randomised data exists for optimal management strategies for men including surgery, systemic therapy, and radiation [22]. Some guidelines have been published for the management of MBC [23,24,25]; however, these guidelines are rarely based on clinical trials leading to a paucity of literature on the evaluation of outcomes for MBC. According to Corrigan et al. [26], of the 131 breast cancer clinical trials conducted, there was only 0.087% of male patients represented among study participants.
Moreover, MBC being widely described as a 'woman’s disease' has psychosocially impacted the experience of men in terms of their body image and appearance as well as masculinity [27, 28]. A critical psychosocial problem for MBC patients is concerns with body image [29], because both the disease and its treatment can lead to significant alterations to their looks and how the body functions [30]. With masculinity often associated with chest rather than breast [31,32,33], being linked to a “woman’s disease” attributed to the body part that men do not relate to is probably threatening their masculinity [34]. Men with breast cancer also face unique physical and emotional challenges however, there is inconclusive understanding of men’s experiences of the psychosocial implications of MBC as well as the supportive care needs [35, 36]. Therefore, in this review, we explored the experiences of MBC patients and the management approaches across different demographic contexts.
Methods
Review question
What are the experiences and perceptions of MBC patients following diagnosis?
Design
We conducted a mixed method systematic review with an interpretive and inductive stance [37] and reported in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [38].
Search strategy
We identified relevant studies through a search in six electronic databases: Global Health, CINAHL, Medline, PsycINFO, Embase, and Web of Science. Furthermore, we searched reference lists of included studies for additional studies. The search duration in these databases covered January 2000 to December 2023, and was updated in September 2023.
A combination of the following keywords was used for search strategy i) ‘Men’ OR ‘Male’ OR ‘Father’ OR ‘Husband’ AND ii) ‘Breast cancer’ OR ‘Breast carcinoma′ OR ‘Breast neoplasm’ OR ‘Breast tumour’ AND iii) ‘Experiences’ OR ‘Perceptions’ OR ‘Perspectives’ OR ‘Opinions’ AND iv) ‘Treatment’ OR ‘Approaches’ OR ‘Outcomes’. Multiple variations of the keywords were used including the truncations based on database requirements to broaden to capture all relevant studies.
Inclusion and exclusion criteria
This review included all primary studies of any design (qualitative, quantitative, or mixed methods) that report on MBC (included only men assigned male gender at birth); studies focussing on the experiences, perceptions, and treatment approaches for MBC; as well as studies conducted and reported in English (based on the resources available to the researchers). However, letters, editorials, commentaries, perspectives, case reports, opinion pieces, news reports and systematic reviews on MBC; studies reporting on cancers in men other than MBC; those that did not report on MBC experiences; as well as those reported in languages other than English were excluded.
Data extraction, quality assessment, synthesis and analysis
Search results were imported into Endnote reference manager (version 20) by the first reviewer (MA-O), duplicates removed and titles as well as abstracts were screened. The remaining studies were screened against the inclusion/ exclusion criteria, by three reviewers (MA-O, JB, OA), and any study for which inclusion was unclear was discussed and resolved by YS and TNA. Full texts studies were obtained if abstracts did not have enough information to determine the relevance of an article. Study variables such as authors, countries where studies were conducted, aims/objectives, study design, sample size and characteristics, experiences of MBC with verbatim quotes, MBC treatment approaches with outcomes and conclusions drawn were extracted to a common table (see Table 1).
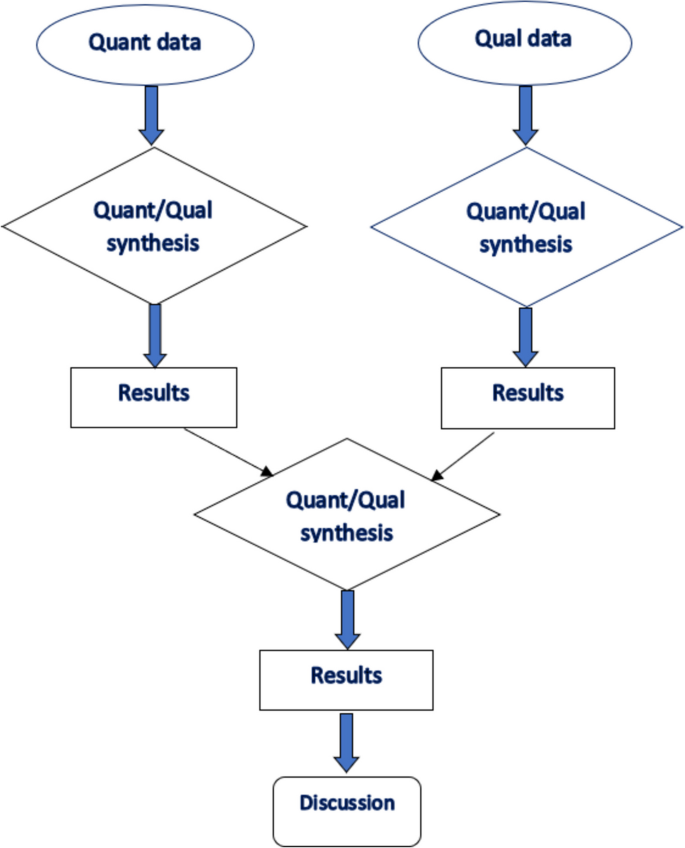
We used a results-based convergent design [75] to guide data analysis, where we initially synthesised qualitative and quantitative findings separately, before integrating these findings from the two designs in the final analysis and synthesis (see Fig. 1). This allowed us to synthesise quantitative findings regarding treatment approaches of MBC and qualitative or mixed methods results on the experiences of MBC patients.
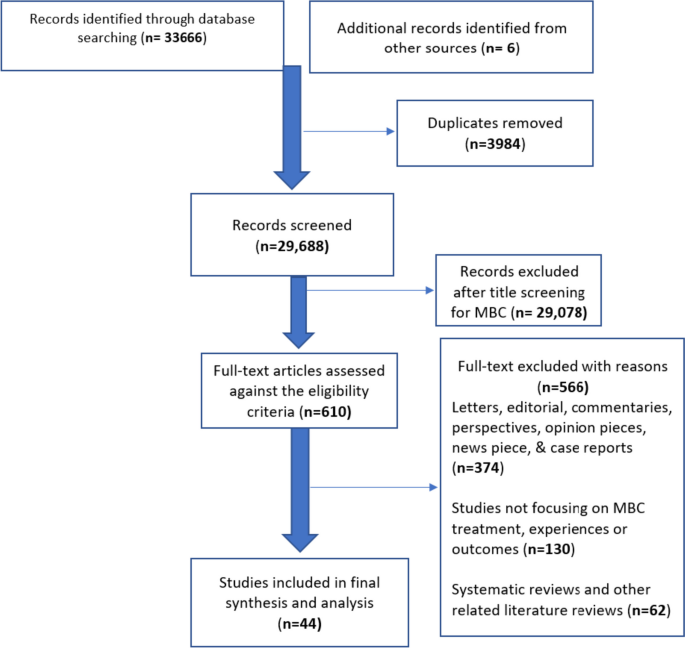
Descriptive statistics was used in reporting the number of published studies and presented in a PRISMA flow diagram in Fig. 2. We synthesised the descriptions of MBC experiences and treatment approaches reported across studies. All studies were analysed descriptively. To synthesise the data regarding the experiences of men with breast cancer, verbatim quotes reported in the qualitative studies were extracted by two authors (JB & TNA). An interpretive and inductive stance was employed [37] by reviewing verbatim quotes to generate codes (see Table 2). Similar codes were aggregated to generate sub-themes followed by formulation of higher order themes. For the quantitative data regarding the treatment modalities, we focused on describing the main reported treatment modalities rather than their frequencies. At the end of the analysis, both the qualitative findings and descriptions from the quantitative studies converged as one dataset. The themes generated from the initial process and the descriptions obtained from the quantitative studies formed the basis of undertaking a narrative synthesis.
The quality of included studies was assessed using the Quality Assessment Tool for Studies with Diverse Designs (QATSDD) tool [76], which is designed for use in mixed methods reviews and quality reporting in reviews that included qualitative, quantitative, mixed- and multi-methods research to ensure consistent and critical appraisal of relevant studies. In assessing study quality, studies were categorised as high quality if they achieved an aggregate score in excess of 70%, moderate quality were assigned to studies scoring between 50 and 70%, and those scoring less than 50% were assigned low quality (see Table 1). However, no study was excluded based on respective aggregate quality scores.
Results
Study characteristics
Of the n = 610 full-text articles assessed for eligibility. N = 374 were excluded as these were letters, editorials, commentaries, perspectives, case reports, opinion pieces and news reports on MBC; including n = 130 studies that did not report on MBC experiences and perceptions; and n = 62 that were MBC related reviews (see Fig. 2). Following extensive search and screening, 44 studies were retained in the final synthesis and analysis, with publication years ranging from January 2000 to September 2023. Twenty-nine studies employed varied quantitative designs, 8 studies employed qualitative designs, and 6 studies employed mixed-method designs. Although most of the studies (n = 44) included only MBC, two retrospective studies compared males and females with breast cancer, and only the data reported on males were included in this review [58, 68]. Study characteristics including quality assessment grading are reported in Table 1.
Experiences and perceptions of males with breast cancer
As shown in Table 2, three themes and nine sub themes emerged from the data which encapsulate the experiences of males with breast cancer.
Theme 1: Navigating through a threat to masculinity and one’s existence
This theme describes how males experienced the illness reflecting on detection, diagnosis, coming to terms with the disease, and disclosure. The subthemes are 1) emergence and awareness of a foreign illness and threat to one’s existence 2) coming to terms with a gendered disease and 3) opening up/ coming out of the illness closet. All included nine qualitative studies highlighted how the affected men perceived breast cancer as a threat to their sense of masculinity.
Emergence and awareness of a foreign illness and threat to one’s existence
Males generally perceived breast cancer as a feminine illness which cannot affect their bodies [31, 34]. In fact, although all the men in the included studies had heard about breast cancer, most of them had not previously heard about breast cancer in males which made them rule out any possibility of ever living with it and may have contributed to delay in seeking healthcare [31, 49]. This perception and the emerging non-specific symptoms often delayed early health seeking as the symptoms were interpreted as irrelevant or not requiring urgent attention [49]. It is worth highlighting that most of the affected men presented with palpable lump in the breast or discharge from the nipple of the affected breast. Some men had to be ‘pushed’ by their wives or partners to seek medical attention to rule out the possibility of breast cancer; a condition they felt was out of their scope [49, 71]. A breast cancer diagnosis was met with varied emotions including being dumbfounded, shocked, surprised, debilitating stress, and a feeling of housing a feminised illness in a masculine body which threatened their sense of masculinity and personhood [13, 31, 34, 49].
“…there is no reason why I shouldn’t have cancer, I’m only the same as anyone else. I’m just a bit disappointed really about where it got me. it’s not right on a man, is it? [31] (p.467).
“From others at work, I always (hear) ‘admit it, you’re just trying to find excuses. You’re not a real man, or you wouldn’t have such an illness’. [34] (p.8).
‘I suppose the fact that it was breast cancer surprised me. The fact that it was cancer I suppose was a shock . . . So, I suppose a combination of both. You know the fact that it was breast cancer which I do not think I had heard of and the fact that it was cancer’’ [13] (p.336).
Receiving the diagnosis was challenging which some men kept to themselves or only informed family/ close friends [71]. The notion of breast cancer being a feminine illness made men view the disease as foreign or exotic to their bodies [49]. The growing awareness of the disease made the men feel a sense of oddity and shame for having a feminine illness alongside a feeling of losing one’s manhood to an illness not considered masculine [31, 49]. Worry, anxiety, and uncertainty also marked their increasing awareness of the disease particularly regarding how the disease could distort the shape of their ‘masculine chest’ [13]. Despite the varied emotions, some males felt extremely lucky that the cancer was located at a site not considered ‘vital’ in terms of masculinity [67].
My biggest problem was how to tell my wife that I have a woman’s disease? Because I thought maybe you’re not a real man, perhaps half woman?” [34] (p.8).
“Now when I first knew that I had it, I thought to myself …well how did Dickens get breast cancer? I’m not a woman. I’m a man. I was surprised more than anything… Women, it's an ever-present threat … Men – never occur to them. ‘‘When I first knew I did not want everyone knowing, because I did not want everyone coming round sympathising’’. [13] (p.336).
Further to the above, the diagnosis of breast cancer forced the affected men to come face to face with their own mortality. This is because they felt a diagnosis of breast cancer threatened their existence and equated to a death sentence. The realisation of death lurking close by pushed the affected men to increase their efforts in attaining their dream before they died. This experience helped them to be more appreciative of their present lives, increased their consciousness about their health, and helped them to redefine their values and beliefs [60]:
“I appreciate life a lot more. Before my cancer, I didn’t take life seriously. I took life for granted. I didn’t appreciate the people in my life and the things I see. So, after the cancer, it was a good kick in the butt. Just how much you appreciate it, and also made me realise to go after my dreams, chase it, and achieve it. Go after it and every day is a gift” [60] (p.3).
Coming to terms with a gendered disease
Through the journey of receiving a breast cancer diagnosis and living with the illness, the affected men expressed the insights and perceptions they gained regarding living with an uncommon illness that is believed to affect mostly women [60]. Following the breast cancer diagnosis, males were faced with the reality of living with a condition they did not expect to have. Coming to terms with a feminised disease was gradual and a lonely journey for the affected men. In fact, some wished they could give their condition another name instead of breast cancer. The fear of being stigmatised made some men keep their diagnosis to themselves [13, 32]. Others also felt a sense of awkwardness discussing such sensitive issues and would avoid [13]. Taken together, men with breast cancer often concealed or attempted to re-label their diagnosis to manage their sense of stigma, shame, and oddity as they navigated through coming to terms with living with a “feminine disease” in their masculine bodies [13, 32, 66]:
‘‘I told the guys I played golf with that I’d got cancer; I do not think so. I necessarily told them it was breast cancer’’. [13] (p.337)
“…but if I did, I would talk about it as chest cancer. I wouldn’t use breast cancer. So that would be the term I would use, and, in the conversation, I would say that it is the same as breast cancer. It’s exactly the same thing; it’s just in my chest.” [66] (p. 964).
“I think among old men they almost consider it to be a stigma, they almost don’t want to tell people, you know, it’s some kind of, I don’t know, a black mark, but I never looked at it that way…I think people younger would just view it a little differently, you know it’s cancer, it’s something they have to deal with, it doesn’t really matter what type of cancer it is.” [67] (p.37)
Opening up/ coming out of the illness closet
As the men gradually came to terms with living with the “foreign or exotic disease”, they were able to talk to their families and close friends about their diagnosis [13]. This required a lot of courage to navigate through such a sensitive issue. Interestingly, the men noted that the process of openly discussing their diagnosis in social spheres and coming out to others offered them an opportunity to reassert the meaning of masculinity, particularly as they recognize how fragile their masculine bodies are [31]:
In two studies, however, the authors described the phenomenon of selective disclosure in which the men only disclosed their illness to selected persons only [20, 60]. For some men, the selective disclosure also meant revealing just the diagnosis, but not going further to reveal how they are experiencing the treatment process or the aftermath of the illness:
“The children know and our closest friends know, the very closest. Why? Because I disappeared for a while. I don’t talk about it within the family, not at all. Nobody talks with me about it, but they know. It is only information, and that’s it, not about the experience and not about the surgery, and not about the treatment” [20] (p.5).
Theme 2: Navigating through treatment
The theme captures the experiences of undergoing breast cancer treatment/ management following their diagnosis. The subthemes are 1) therapeutic interventions 2) navigating through feminised treatment pathways and 3) living with the effects of care/ ongoing treatment. All included qualitative, quantitative, and mixed method studies (n = 44) highlighted the treatment experiences and pathways respectively.
Therapeutic interventions
Several therapeutic interventions/ treatments were reported across the included studies. Five categories of treatments were ascertained across the included studies, and these are surgery, radiotherapy, chemotherapy, hormonal therapy, and palliative care. Surgical interventions included mastectomy with axillary dissection, mastectomy with sentinel node biopsy (both for men with late-stage breast cancer presentation), and lumpectomy [7, 40, 45,46,47,48]. Cronin et al., [46] noted that surgery and chemotherapy receipt were more likely among men up to age 65. In some studies, surgical interventions were the main forms of treatment with radiotherapy, chemotherapy, and hormone therapy playing adjuvant roles. For instance, in one study that included 37 men with breast cancer, radiotherapy (89.2%), hormonal therapy (56.7%), and chemotherapy (91.8%) were adjuvant therapies after surgery [48]. In one study, the authors reported several therapeutic regimens offered to men with breast cancer which included breast conserving surgeries, unilateral/ bilateral mastectomy, often with no reconstruction [44]. One third of the male breast cancer patients in the same study (n = 21) felt somewhat or very uncomfortable with their appearance after the surgery. Receipt of treatment was remarkably similar between blacks and whites in both age groups. Older black and white men had lower receipt of chemotherapy (39.2% and 42.0%, respectively) compared with younger patients (76.7% and 79.3%, respectively). Younger black men had a 76% higher risk of death than younger white men after adjustment for clinical factors only (HR, 1.76; 95% CI, 1.11 to 2.78), but this difference significantly diminished after subsequent adjustment for insurance and income (HR, 1.37; 95% CI, 0.83 to 2.24). In those age 65 years, the excess risk of death in blacks versus whites was nonsignificant and not affected by adjustment for covariates.
Navigating through feminised treatment pathways
Despite the reality of breast cancer among males, the care pathways and healthcare payment frameworks across various healthcare systems are significantly tailored to the needs of females which reinforces the notion of the disease as a feminine in nature [31, 71]. A study from Germany highlighted the difficulty that these men experience in finding a physician as the practitioners felt their breast care specialty targeted women and would lose on reimbursement [34]. Even in facilities where they were given satisfactory care, the men felt the services and procedures still failed to consider their unique needs as men with breast cancer [31, 42, 71]. Some men were mistakenly addressed as females on the assumption that only females experienced breast cancer [34]. Male-specific psychosocial support and information were generally lacking across the studies. Information leaflets mostly contained pictures of female breast cancer patients which made the men feel excluded [34]. In fact, they felt the service was not designed for them:
“My GP said: ‘I don’t know what to do any more, it’s not my specialty area. I’ll have to refer you to someone else’. And the other doctor said, ‘This is a women’s practice (…) and we can’t get reimbursed for men, we don’t want men here.’” [34] (p.9).
‘‘. . . but I think as a male the information that I was given was female orientated and it could have been better presented for me and . . .I know that every case is different, but it was lacking in that respect’’. [13] (p.336).
Further to the above, some men had several challenges in scheduling for therapeutic regimen such as mammography [67]. Interactions with healthcare providers were often considered awkward as the providers often did not know what to say to the men with breast cancer. Subsequently, most men with breast cancer undergoing treatment often felt like outsiders, out of place, marginalised, and alone:
‘No information. Nothing at all. It was like men; you are on your own. I daresay women aren’t left like that . . .On leaving after the first operation the nurse gave me a leaflet, a piece of paper with women on it doing exercises you have to do and that was it’’. [13] (p.336).
“I find that dealing with the mammograms and the technical staff to kind of tiptoe around you and put you in certain places because they don’t expect a male to be there, right, so they got women walking around in their gowns, so they don’t want you in those areas… they kind of shunt you into an isolated, a more isolated area so you’re not seeing the women walking by.” [66] (p.967).
Living with the effects of care/ ongoing treatment
Men undergoing treatment for breast cancer felt their lives, roles, and occupations were impacted adversely by the treatment regimen [60]. The clinical management process of the disease, in fact, further heightened the gendered essence of the disease. For men who underwent surgical intervention, the mastectomy scar served as a permanent reminder of the disease impacted on their masculinity [66]. Others felt their chest had deformed due to the scar [71]. The typical exposure of the male chest at leisure activities such as the beach was considered a no-go area to conceal the scar from public view. The scars also evoked a sense of perceived stigma among these men [32]:
“I’ve been abroad and sunbathed. People do look, they do look” [71] (p.1835).
“I don’t feel like a complete person either because I’ve got something missing, haven’t I? ... My nipples are not there anymore. Sometimes I look in the mirror . . . I don’t like doing that. It’s gone. . . There’s a scar across there. . .Doctor said I look like a patchwork quilt. So, I don’t bother taking my shirt off now. And something else … yes you ought to have a tattoo as a nipple’’. [13] (p.337).
For men who underwent hormone therapy, it was observed that the side effects of the various medications threatened their notion of being a male. Experiencing erectile dysfunction and loss of libido were really challenging for these men as they felt they had lost their sense of masculinity or what made them men [34, 77]. Hair loss from chemotherapy was also challenging and frustrating for them [43]. These men felt as though they had been transformed to ‘menopausal women’ [34].
“We’re candid and honest with one another … male sexual potency has gone.” [34] (p.9).
“This has killed my sex life; I can no longer get an erection. I’m on this Tamoxifen which I’ve got to take for 5 years. You know it’s driving me mad. I get free Viagra but there is nothing there. There are no feelings or anything like that and it’s terrible. I couldn’t get an erection or nothing. I don’t know what it was, I just felt so no, no (silence) I just felt so embarrassed.” [31] (p.467).
Further to the above, some men felt they were a burden to others as they had to rely on others to have their needs met. Younger males felt their traditional roles as providers of the family was threatened as their dependence increased with a slow return to work and had to be supported by their spouses [54]:
“You start to receive only sickness benefits and when all of a sudden, you have over 500 euro less, you have to first see how you manage with that. And for me [...] it was even more because I only have a 60% part-time job and work as a freelancer on the side. And that I couldn't do any longer either.” [54] (p.6).
Theme 3: Coping and support systems
The theme describes how men with breast cancer coped with the disease, treatment process, aftercare/ rehabilitative care, and the available support and it was reported across qualitative (n = 9), quantitative (n = 5) and mixed methods (n = 4) studies. The subthemes are 1) active coping strategies 2) family support and 3) support from healthcare providers and other support groups.
Active coping strategies
Although the breast cancer diagnosis was considered threatening with intense emotional stress, some affected men remained optimistic and hopeful of improved outcomes. Affected men often worked towards accepting the disease which made the navigation process less challenging [47]. The treatment process and aftercare phase offered the affected men an opportunity to amend or reformulate their notion of masculinity [66]. Although dealing with the disease was difficult, the men reportedly gained new insights in life which helped to reshape their worldviews and life priorities [14]. In addition, previous experience with breast cancer in the family was associated with use of non-repressing coping styles (X2[1, N = 26] r = 5.60, p < 0.05). There was also a higher use of mature defence patterns (superior healthy neurotic functioning) in patients who use non-repressive coping [70]. Despite the identified active coping mechanisms, one study reported that majority (70%) of men with breast cancer used immature and neurotic defensive functioning and 53.8% used a repressive approach to bottle up their emotions and concerns and [70]:
“I was kind of self-conscious the first year or so but um, I’m in pretty good shape, I’m relatively muscular, not super muscular, but I’m toned, I’m in shape, and I think a lot of times unless I’m really up close to people, I think a lot of times they don’t even see it… I’m not self-conscious. I go on vacation or go swimming at the beach, I don’t feel like people are staring at me.” [67] (p.38)
“Breast cancer, for me, means a whole complex of experiences, of realisations. It’s like being in the military, you know. You meet somebody who’s been in the military, you don’t have to say anything. But if you meet someone who hasn’t, there’s not a way in the world to describe what it’s like.” [67] (p.38)
Family support
Studies found that majority of patients (61.3–80%) disclosed and discussed their diagnosis with their spouses and close families while 4–21% refused to disclose or discuss with anyone [7, 13, 61]. This might be because less stigmatization was reported from close families and friends compared to broader social settings [32]. Such disclosure might also be protective as availability of marital support was found to influence treatment choice and outcomes. Men who were not currently married received chemotherapy significantly less often [52] and had significantly higher (in some cases up to 21%) mortality than married ones [52, 53].
This was corroborated by included qualitative studies which reported on the family support that men affected with breast cancer received. Spousal support was identified as a significant resource to seeking healthcare in the first instances as some wives had to push their partners to seek medical care [31, 57]. Spousal and family support also helped men to navigate through the breast cancer diagnosis, coming to terms with the disease [49, 57]. Family support was also an essential resource during the treatment and aftercare phase as family members offered emotional and practical support [47]:
“My wife was my support – she and I talked about everything. At the beginning we talked about it and agreed that I would have her as my support and she would have her family to support her through. It worked well and I also got support from her family . . . mine were useless’’. [13] (p. 338).
Support from healthcare providers and other support groups
Studies reported the dimensions, contents and timing of information needs demonstrated by the patients. Men with breast cancer acknowledged the support received from healthcare providers regarding diagnosis, information, treatment options, and aftercare support [49, 57] with the most common source of information being verbal (92%), leaflets or booklets (53–71%) and internet (20%) [61]. Yet, 36–65% of participants felt their needs were not always met and wanted more information on various contents (particularly sexuality related information) at different times in their treatment (early/acute effects, late effects and ongoing quality of life) and in a more male specific manner [42].
Men with Breast cancer faced challenges in accessing needed support from healthcare facilities. Included studies reported experience of embarrassment and stigmatization within healthcare facilities where male breast cancer patients were meant to get support. 51.6% of patients experienced "extreme" or "very" severe embarrassment while waiting in the clinic among other female patients [13]. The experience of stigmatization was found to be higher within the cancer care system than other social surroundings with significantly higher stigmatization incidences reported in rehabilitation settings (mean = 1.50) and during hospitalisations (mean = 1.20) [53].
A mixed finding was observed regarding usage of peer supports. For one-to-one peer support, Iredale et al. (2006) reported low utilisation of formal support services with only 19% of participants speaking to other men who had breast cancer and only 1 in 4 indicating they would have liked that opportunity after their diagnosis. However, Midding et al. [53] found that more men (63.2%) had a one-on-one peer support from a female Breast Cancer Patient compared to 24.2% from another male breast cancer patient. This is consistent with the qualitative data which showed some men appreciated the opportunity to talk to other men with breast cancer on one-to-one basis [34, 71], other men did not prefer this and were satisfied with the support offered by the healthcare providers and their families [13]:
‘‘…none of the guys wanted to have self-help groups ... I don’t think they need the psychological support that perhaps women do, and women tend to congregate and talk about these things anyway. I think this is, of course ... research I know ... but actually quite therapeutic in a way’’. [13] (p.338).
“To be honest, I don’t know how I would be managing if I had never had (the support group). They gave me back the will to live and I will always be grateful for that.” [43] (p. 9).
In terms of group peer support, studies reported that only 15.3% of the participants were part of a peer support group and majority (96.3%) of participants who were not currently part of a support group did not wish to be part of a support group whether male only or mixed sex [53, 61].
Discussion
Breast cancer is generally perceived to be a disease common among women albeit incidence among men is slowly rising, creating a need for health systems to be responsive to their needs. To this end, this review sought to develop a comparative understanding of the experiences of men with breast cancer and the treatment options available to them across different demographic settings. The review findings highlight the embodiment of breast cancer as a ‘feminine’ disease which is incongruent with what it means to be a ‘man’ and hegemonic masculinity discourses. Throughout the trajectory of the disease (that is, from diagnosis to aftercare), the review findings underscore the gendered nature of the disease with a lack of health system preparedness to support men who develop a disease perceived to be ‘feminine’. Though the treatment pathways were similar to those observed in the management of female breast cancer patients, they do not necessarily meet the unique needs of MBC across the disease trajectory warranting urgent attention considering the increasing prevalence of the disease among men. Male-specific treatment pathways, ongoing education, and professional support are also required.
The breast is seen as a symbol of femininity, and as incongruent with being male, together with the significant public health emphasis on the prevention of breast cancer among females [78, 79] have further championed the perception that breast cancer is a feminine illness [56, 67]. Thus, it was not surprising that the finding regarding being out of sync with one’s body resonated across the included studies. The breast cancer diagnosis which commenced the illness trajectory was really challenging for the men and filled with varied emotions. Despite the difficulty, the professional support available was often gendered and unsuitable to their needs. Thus, they mostly had to rely on their spouses and close families/ friends if they were able to open up to them, which may take some time. Coupled with the hegemonic masculinity ideology that a man must always be in charge and not demonstrate any emotions which can be perceived as weakness, it is likely that men will navigate through these on their own which can make the journey very lonely for them. Agreeing with a previous study, depressive symptoms, anxiety, and traumatic stress symptoms were common occurrences following the breast cancer diagnosis [43]. The culture of silence around the issue can lead to utilising avoidant coping mechanisms which may delay support seeking among men. Taken together, the findings highlight a need for tailor-made, individualised counselling support service for men before, during, and after breast cancer diagnosis. The need for healthcare professionals to consider the impact of the MBC on men cannot, therefore, be overemphasised.
Commencing treatment and aftercare/ rehabilitative support is an equally challenging phase for men living with breast cancer. A previous study has observed that gender impacts on the experience with breast cancer treatment [15]. The review findings highlighted the ‘feminised’’ nature of the treatment pathways with some practitioners not even knowing how to support the affected men. Information leaflets and other educational materials were generally noted to be filled with images of females which made the men feel out of place. Overall, these can serve as structural barriers which potentially deter men from seeking help even when required [34]. Undoubtedly, breast cancer affects more females than males. However, healthcare service delivery should be tailored to the unique needs of men to overcome the feeling of marginalisation or being left out. The impact of the therapeutic regimen should also be highlighted particularly as they can lead to loss of libido or erectile dysfunction which further diminishes one’s sense of being a man in relation to societal norms. Surgical procedures can lead to scars which serve as permanent reminders of the illness which can have life-long impact on men. Professional support should therefore not end after the diagnosis phase but should extend to the entire treatment continuum and aftercare. There is also a need to raise awareness of male breast cancer among healthcare practitioners to improve their approach to individuals through person-centred and male-specific care strategies. It may be worth reiterating the recommendation by Nguyen et al., [34] suggesting a guideline targeting men with breast cancer to support healthcare practitioners in the health and social service delivery process.
The need for support was reiterated throughout the review, and this is corroborated in a previous study where family and spousal support was critically important for men with advanced prostate cancer [80]. Interestingly, mixed findings were observed regarding the need for male-specific support groups. Although this may be based on individual preferences, it may also emanate from the hegemonic masculinity ideology [80, 81] or coping styles such as disengagement [20] as men may appear ‘stoic’ in the presence of such difficult moments and may not want to seek help [34, 82]. A breast cancer diagnosis can profoundly impact masculinity, with men grappling with navigating a threat to masculinity which collectively challenges one's sense of self and traditional gender roles [82,83,84].
Recent research shows changing perceptions of breast cancer as a "feminine disease" due to awareness campaigns and shifts in societal attitudes [85, 86]. Additionally, demographic factors like location of treatment, socioeconomic status, and age have been found to affect the quality of care and outcomes, while acknowledging the male breast cancer experience and its shared emotional aspects with women's experiences [87, 88]. These highlights evolving healthcare practices and societal norms regarding breast cancer.
Despite this, it is still cogent to understand their lived experiences and advocate for men support groups, if they would like to join one, as they navigate through the diagnosis, treatment, and aftercare pathway. This study presents the synthesis of multicultural evidence to highlight the cross-cultural similarity in the reaction and lived experience of men when faced with the diagnosis of breast cancer.
Strengths and limitations
The strength of this mixed method is the inclusion of studies from different countries and settings in addition to including and synthesising studies on the experiences of patients with male breast cancer from diagnosis to aftercare. Notwithstanding, there are some limitations that need to be highlighted. Firstly, a real limitation of our review was including only studies published in English. Excluding studies that used a language other than English, potentially led to information loss that could come from relevant studies written in other languages and restricts this mixed methods review only to the views and perception of men living in English speaking countries or countries where practitioners write and publish in English. Secondly, we acknowledge that younger and older men may have unique experiences while navigating breast cancer diagnosis and treatment. These nuances were not captured in the current review and may be worth exploring in future studies.
Conclusion
Men experience a myriad of issues following a breast cancer diagnosis, underscored by their ideology of masculinity. Our findings suggest the need for healthcare professionals’ training and education on managing interactions with MBC patients in a way that does not propagate a sense of awkwardness and otherness in a feminised support structure. Additionally, policy must address the structural barriers to treatment access for MBC including healthcare finance reimbursements that limit access to gendered specialist breast cancer treatments. Awareness creation efforts of MBC among the public as well as healthcare practitioners are urgently required to explain to the public through television programmes and awareness meetings that breast cancer is a disease like any other that affects both men and women. Creating such awareness could lead to changing the perception of men and promote early diagnosis, adherence to treatments, post-treatment monitoring, oncological results, and a better quality of life. Professional care intervention and support for MBC should not end after the diagnosis phase but should extend to the entire treatment continuum and aftercare. Preserving sexual function is an important finding highlighted from this review. Research will be needed to develop and test testosterone-preserving treatment modalities or optimising existing therapies in a way that is relevant to the priorities of MBC. This will also require the development of specialised guidelines for healthcare practitioners on MBC to optimise care and treatment for MBCs in a person-centred manner as suggested by other studies. To develop such individualised support frameworks, it is imperative to understand the specific needs, priorities, and support preferences among MBC patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Society AC. Key Statistics for Breast Cancer in Men 2022 [Available from: https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html.
Chen Z, Xu L, Shi W, Zeng F, Zhuo R, Hao X, et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990–2017. Breast Cancer Res Treat. 2020;180(2):481–90.
Health UDo, Services H. Making Cancer Health Disparities History: Report of the Trans-HHS Cancer Health Disparities Progress Review Group. Washington, DC: U.S. Department of Health and Human Services; 2004.
Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37.
Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105–13.
Rhoads KF, Cullen J, Ngo JV, Wren SM. Racial and ethnic differences in lymph node examination after colon cancer resection do not completely explain disparities in mortality. Cancer. 2012;118(2):469–77.
Co M, Lee A, Kwong A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med. 2020;9(10):3305–9.
Ndom P, Um G, Bell EMD, Eloundou A, Hossain NM, Huo D. A meta-analysis of male breast cancer in Africa. The Breast. 2012;21(3):237–41.
Fiala L, Coufal O, Fait V, Foretova L. Male breast cancer-our experience. Rozhledy v chirurgii: mesicnik Ceskoslovenske chirurgicke spolecnosti. 2010;89(10):612–8.
Rudlowski C. Male breast cancer. Breast care. 2008;3(3):183–9.
Robinson JD, Metoyer KP, Bhayani N. Breast cancer in men: a need for psychological intervention. J Clin Psychol Med Settings. 2008;15(2):134–9.
Thomas E. Men’s awareness and knowledge of male breast cancer. AJN The American Journal of Nursing. 2010;110(10):32–7.
Iredale R, Brain K, Williams B, France E, Gray J. The experiences of men with breast cancer in the United Kingdom. Eur J Cancer. 2006;42(3):334–41.
Pituskin E, Williams B, Au H-J, Martin-McDonald K. Experiences of men with breast cancer: A qualitative study. Journal of Men’s Health and Gender. 2007;4(1):44–51.
Naymark P. Male breast cancer: incompatible and incomparable. Journal Of Men’s Health And Gender. 2006;3(2):160–5.
Ottini L, Palli D, Rizzo S, Federico M, Bazan V, Russo A. Male breast cancer. Crit Rev Oncol Hematol. 2010;73(2):141–55.
Rudan I, Rudan N, Basić N, Basić V, Rudan D. Differences between male and female breast cancer. II. Clinicopathologic features. Acta Medica Croatica. 1997;51(3):129–33.
Sineshaw HM, Freedman RA, Ward EM, Flanders WD, Jemal A. Black/white disparities in receipt of treatment and survival among men with early-stage breast cancer. J Clin Oncol. 2015;33(21):2337–44.
DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–33.
Levin-Dagan N, Baum N. Passing as normal: Negotiating boundaries and coping with male breast cancer. Soc Sci Med. 2021;1(284):114239.
Sousa B, Moser E, Cardoso F. An update on male breast cancer and future directions for research and treatment. Eur J Pharmacol. 2013;717(1–3):71–83.
Fentiman IS. Surgical options for male breast cancer. Breast Cancer Res Treat. 2018;172:539–44.
Cardoso F, Bartlett J, Slaets L, Van Deurzen C, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29(2):405–17.
Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114.
Miao H, Verkooijen H, Chia K-S, Bouchardy Magnin C, Pukkala E, Larønningen S, et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29(33):4381–6.
Corrigan KL, Mainwaring W, Miller AB, Lin TA, Jethanandani A, Espinoza AF, Piotrowski M, Fuller CD, Stauder MC, Shaitelman SF, Perkins GH. Exclusion of men from randomized phase III breast cancer clinical trials. Oncologist. 2020;25(6):e990–2.
Manier KK, Rowe LS, Welsh J, Armstrong TS. The impact and incidence of altered body image in patients with head and neck tumors: a systematic review. Neuro-Oncology Practice. 2018;5(4):204–13.
Himmelstein MS, Sanchez DT. Masculinity impediments: Internalized masculinity contributes to healthcare avoidance in men and women. J Health Psychol. 2016;21(7):1283–92.
Paraskeva N, Clarke A, Harcourt D. Altered appearance from cancer. Body image care for cancer patients: Principles and practices. 2018;3:131–60.
Fingeret MC, Teo I, Epner DE. Managing body image difficulties of adult cancer patients: lessons from available research. Cancer. 2014;120(5):633–41.
Donovan T, Flynn M. What makes a man a man?: The lived experience of male breast cancer. Cancer Nurs. 2007;30(6):464–70.
Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman’s disease”: Stigmatization of male breast cancer patients—A mixed methods analysis. Am J Mens Health. 2018;12(6):2194–207.
Pituskin E, Williams B, Au HJ, Martin-McDonald K. Experiences of men with breast cancer: A qualitative study. Journal of Men’s Health and Gender. 2007;4(1):44–51.
Nguyen TS, Bauer M, Maass N, Kaduszkiewicz H. Living with male breast cancer: a qualitative study of men’s experiences and care needs. Breast Care. 2020;15(1):6–13.
Younas A. Epistemic injustice in health care professionals and male breast cancer patients encounters. Ethics Behav. 2021;31(6):451–61.
Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918–26.
Elbardan H, Kholeif AO, Elbardan H, Kholeif AO. An interpretive approach for data collection and analysis. Enterprise Resource Planning, Corporate Governance and Internal Auditing: An Institutional Perspective. 2017:111–65.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann intern med. 2009;151(4):264–9.
Adekolujo OS, Tadisina S, Koduru U, Gernand J, Smith SJ, Kakarala RR. Impact of marital status on tumor stage at diagnosis and on survival in male breast cancer. Am J Mens Health. 2017;11(4):1190–9.
Ahmed A, Ukwenya Y, Abdullahi A, Muhammad I. Management and outcomes of male breast cancer in Zaria. Nigeria Int J Breast Cancer. 2012;2012:845143.
Avila J, Herrick B, Attai DJ, Leone JP. Treatments for breast cancer in men: late effects and impact on quality of life. Breast Cancer Res Treat. 2023;201(3):489–98.
Bootsma TI, Duijveman P, Pijpe A, Scheelings PC, Witkamp AJ, Bleiker EM. Unmet information needs of men with breast cancer and health professionals. Psychooncology. 2020;29(5):851–60.
Brain K, Williams B, Iredale R, France L, Gray J. Psychological distress in men with breast cancer. J Clin Oncol. 2006;24(1):95–101.
Chichura A, Attai DJ, Kuchta K, Nicholson K, Kopkash K, Pesce C, Yao K. Male breast cancer patient and surgeon experience: the male WhySurg study. Ann Surg Oncol. 2022;29(10):6115–31.
Crew KD, Neugut AI, Wang X, Jacobson JS, Grann VR, Raptis G, et al. Racial disparities in treatment and survival of male breast cancer. J Clin Oncol. 2007;25(9):1089–98.
Cronin PA, Romanoff A, Zabor EC, Stempel M, Eaton A, Smyth LM, et al. Influence of age on the clinical outcome of breast cancer for men and the development of second primary cancers. Ann Surg Oncol. 2018;25(13):3858–66.
Duarte do Amaral DE, Manfrim Muniz R, Habekost Cardoso D, Tuerlinckx Noguez P, Ferreira Fagundes R, Costa Viegas A. Male breast cancer: the survivor's context. J Nurs UFPE/Revista de Enfermagem UFPE. 2017;11(5).
El-Beshbeshi W, Abo-Elnaga EM. Male breast cancer: 10-year experience at mansoura university hospital in Egypt. Cancer Biol Med. 2012;9(1):23.
France L, Michie S, Barrett-Lee P, Brain K, Harper P, Gray J. Male cancer: a qualitative study of male breast cancer. The Breast. 2000;9(6):343–8.
Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, et al. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104(11):2359–64.
Halbach SM, Midding E, Ernstmann N, Würstlein R, Weber R, Christmann S, et al. Male breast cancer patients’ perspectives on their health care situation: a mixed-methods study. Breast Care. 2020;15(1):22–9.
Harlan LC, Zujewski JA, Goodman MT, Stevens JL. Breast cancer in men in the United States: a population-based study of diagnosis, treatment, and survival. Cancer. 2010;116(15):3558–68.
Hill TD, Khamis HJ, Tyczynski JE. Comparison of male and female breast cancer incidence trends, tumor characteristics, and survival. Ann Epidemiol. 2005;15(10):773–80.
Hiltrop K, Heidkamp P, Halbach S, Brock-Midding E, Kowalski C, Holmberg C, et al. Occupational rehabilitation of male breast cancer patients: Return patterns, motives, experiences, and implications—A qualitative study. Eur J Cancer Care. 2021;30(4): e13402.
Hoffman A, Horesh N, Shabtai M, Forschmidt E, Rosin D, Gutman M, et al. Breast Cancer in Men: A Single Center Experience Over a Period of 22 years. The Israel Medical Association Journal: IMAJ. 2020;22(3):160–3.
Kowalski C, Steffen P, Ernstmann N, Wuerstlein R, Harbeck N, Pfaff H. Health-related quality of life in male breast cancer patients. Breast Cancer Res Treat. 2012;133(2):753–7.
Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Social support of male breast cancer patients—a mixed-methods analysis. Am J Mens Health. 2019;13(4):1557988319870001.
Nahleh ZA, Srikantiah R, Safa M, Jazieh AR, Muhleman A, Komrokji R. Male breast cancer in the veterans affairs population: a comparative analysis. Cancer. 2007;109(8):1471–7.
Özkurt E, Tükenmez M, Yılmaz R, Cabioğlu N, Müslümanoğlu M, Dinççağ AS, et al. Favorable long-term outcome in male breast cancer. European Journal of Breast Health. 2018;14(3):180.
Potter AM, Bentz B, Crue L, Leiby S, Bashi S, Maguire K, Meyers J, Mieczkowski K. Men’s Lived Experiences of Breast Cancer and Changes in Occupation. Occup Ther Int. 2023;13:2023.
Rayne S, Schnippel K, Thomson J, Reid J, Benn C. Male breast cancer has limited effect on survivor’s perceptions of their own masculinity: a record review and telephone survey of patients in Johannesburg, South Africa. Am J Mens Health. 2017;11(2):246–52.
Sanguinetti A, Polistena A, Lucchini R, Monacelli M, Galasse S, Avenia S, et al. Male breast cancer, clinical presentation, diagnosis and treatment: Twenty years of experience in our Breast Unit. Int J Surg Case Rep. 2016;20:8–11.
Sarmiento S, McColl M, Musavi L, Gani F, Canner JK, Jacobs L, et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res Treat. 2020;180(2):471–9.
Shah S, Bhattacharyya S, Gupta A, Ghosh A, Basak S. Male breast cancer: a clinicopathologic study of 42 patients in eastern India. Indian J Surg Oncol. 2012;3(3):245–9.
Shin JY, Kachnic LA, Hirsch AE. The impact of race in male breast cancer treatment and outcome in the United States: a population-based analysis of 4,279 patients. Int J Breast Cancer. 2014;2014:685842.
Skop M, Lorentz J, Jassi M, Vesprini D, Einstein G. “Guys don’t have breasts”: The lived experience of men who have BRCA gene mutations and are at risk for male breast cancer. Am J Mens Health. 2018;12(4):961–72.
Thompson EH Jr, Haydock AS. Men’s lived experiences with breast cancer: The double consciousness of marginal men. Sex Roles. 2020;82(1–2):28–43.
Visram H, Kanji F, Dent S. Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol. 2010;17(5):17–21.
Wang Y, Chen K, Yang Y, Tan L, Chen L, Zhu L, et al. Incidence and survival outcomes of early male breast cancer: a population-based comparison with early female breast cancer. Ann transl med. 2019;7(20):536.
Weber R, Ehrenthal JC, Brock-Midding E, Halbach S, Würstlein R, Kowalski C, Ernstmann N. Defense mechanisms and repressive coping among male breast cancer patients. Front Psych. 2021;10(12): 718076.
Williams B, Iredale R, Brain K, France E, Barrett-Lee P, Gray J. Experiences of men with breast cancer: an exploratory focus group study. Br J Cancer. 2003;89(10):1834–6.
Yadav BS, Sharma SC, Singh R, Dahiya D, Ghoshal S. Male breast cancer: Outcome with adjuvant treatment. J Cancer Res Ther. 2020;16(6):1287.
Yoney A, Kucuk A, Unsal M. Male breast cancer: a retrospective analysis. Cancer/Radiothérapie. 2009;13(2):103–7.
Zongo N, Ouédraogo S, Korsaga-Somé N, Somé OR, Go N, Ouangré E, et al. Male breast cancer: diagnosis stages, treatment and survival in a country with limited resources (Burkina Faso). World journal of surgical oncology. 2018;16(1):1–7.
Hong QN, Pluye P, Bujold M, Wassef M. Convergent and sequential synthesis designs: implications for conducting and reporting systematic reviews of qualitative and quantitative evidence. Syst Rev. 2017;6(1):1–14.
Sirriyeh R, Lawton R, Gardner P, Armitage G. Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract. 2012;18(4):746–52.
Ruddy KJ, Giobbie-Hurder A, Giordano SH, Goldfarb S, Kereakoglow S, Winer EP, et al. Quality of life and symptoms in male breast cancer survivors. The Breast. 2013;22(2):197–9.
Dozier R. Beards, breasts, and bodies: Doing sex in a gendered world. Gend Soc. 2005;19(3):297–316.
Sulik GA. Pink ribbon blues: How breast cancer culture undermines women’s health. Walton Street, Oxford: Oxford University Press; 2010.
Salifu Y, Almack K, Caswell G. ‘My wife is my doctor at home’: A qualitative study exploring the challenges of home-based palliative care in a resource-poor setting. Palliat Med. 2021;35(1):97–108.
Connell RW, Messerschmidt JW. Hegemonic masculinity: Rethinking the concept. Gend Soc. 2005;19(6):829–59.
Salifu Y, Almack K, Caswell G. ‘Out of the frying pan into the fire’: a qualitative study of the impact on masculinity for men living with advanced prostate cancer. Palliative Care and Social Practice. 2023;17:26323524231176828.
Quincey K, Williamson I, Wildbur D. Men with breast cancer and their encounters with masculinity: An interpretative phenomenological analysis using photography. Psychology of Men & Masculinities. 2021;22(4):690.
Quincey K, Williamson I, Winstanley S. ‘Marginalised malignancies’: A qualitative synthesis of men’s accounts of living with breast cancer. Soc Sci Med. 2016;149:17–25.
Co M, Lee A, Kwong A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med. 2020;9(10):3305–9.
Nguyen TS, Bauer M, Maass N, Kaduszkiewicz H. Living with male breast cancer: a qualitative study of men’s experiences and care needs. Breast Care. 2020;15(1):6–13.
Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, Ruddy KJ. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126(1):26–36.
Wang F, Shu X, Meszoely I, Pal T, Mayer IA, Yu Z, Shu XO. Overall mortality after diagnosis of breast cancer in men vs women. JAMA oncol. 2019;5(11):1589–96.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
This review was conceived and designed by JB, MA-O, YS, OA, and TNA. The first reviewer imported all search results to Endnote reference manager version X9, de-duplicated, then all authors screened titles and abstracts of all identified studies, any article for which inclusion was unclear were discussed and if necessary adjudicated by YS and TNA. All authors critically appraised and contributed to the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abboah-Offei, M., Bayuo, J., Salifu, Y. et al. Experiences and perceptions of men following breast cancer diagnosis: a mixed method systematic review. BMC Cancer 24, 179 (2024). https://doi.org/10.1186/s12885-024-11911-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-11911-9