- Research
- Open access
- Published:
Final analysis of the phase 3 randomized clinical trial comparing HD201 vs. referent trastuzumab in patients with ERBB2-positive breast cancer treated in the neoadjuvant setting
BMC Cancer volume 23, Article number: 112 (2023)
Abstract
Background
The TROIKA trial established that HD201 and trastuzumab were equivalent in terms of primary endpoints (total pathological complete response) following neoadjuvant treatment. The objective of the present analysis was to compare survival outcomes and final safety.
Methods
In the TROIKA trial, patients with ERBB2-positive early breast cancer were randomized and treated with either HD201 or the referent trastuzumab. Eligible patients received 8 cycles of either HD201 or referent trastuzumab (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) every 3 weeks in combination with 8 cycles of chemotherapy (4 cycles of docetaxel, 75 mg/m2, followed by 4 cycles of epirubicin, 75 mg/m2, and cyclophosphamide, 500 mg/m2) in the neoadjuvant setting. The patients then underwent surgery followed by 10 cycles of adjuvant HD201 or referent trastuzumab according to their initial randomization to complete one year of trastuzumab-directed therapy. Event-free and overall survival rates were calculated using Kaplan–Meier analysis. The hazard ratio for event-free survival was estimated by Cox proportional hazards regression.
Results
The final analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). A total of 502 randomized patients received either HD201 or the referent trastuzumab, and 474 (94.2%) were eligible for inclusion in the per-protocol set. In this population, the 3-year event-free survival rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (HR 1.02, 95% CI: 0.63–1.63; p = 0.945). The 3-year overall survival rates were comparable between the HD201 (95.6%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606). During the posttreatment follow-up period, adverse events were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the reference trastuzumab groups, respectively. Serious adverse events were rare and none of which were related to the study treatment.
Conclusions
This final analysis of the TROIKA trial further confirms the comparable efficacy and safety of HD201 and trastuzumab.
Trial registration
ClinicalTrials.gov identifier: NCT03013504.
Introduction
In the primary analysis of the prospective, randomized, multicenter phase 3 TROIKA study, HD201, a trastuzumab biosimilar, was shown to be equivalent to the referent trastuzumab in patients with ERBB2-positive early breast cancer (EBC) based on the primary endpoints of locally assessed total pathologic complete response (tpCR) [1].
The relationship between tpCR status and survival has been extensively debated following a meta-analysis indicating that tpCR status predicts survival outcome in patients with ERBB2-positive EBC [2]. Regulatory agencies have acknowledged this relationship by authorizing several compounds on this early criterion for activity [3,4,5,6,7]. The neoadjuvant setting can be definitively considered the new era for development in ERBB2-positive breast cancer [8]. It remains reassuring that in most cases, the conclusion derived from the early criteria of pathologic complete response (pCR) has been confirmed by survival outcome analysis [9,10,11]. In this final analysis of the TROIKA study, we report the long-term efficacy and safety outcomes at 3 years of follow-up.
Methods
Study design and patients
TROIKA (NCT03013504) was a multicenter, randomized, phase 3 trial previously detailed in the publication reporting the primary analysis [1]. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Approval of the study protocol and all accompanying documents provided to the patients was obtained from independent ethics committees at participating institutions, and all patients provided voluntary written informed consent. Key eligibility criteria were age ≥ 18 years; ERBB2-positivity; new diagnosis; unilateral, operable breast cancer; and a baseline left ventricular ejection fraction ≥ 55%.
Patients were enrolled and randomized using a block of 8 in a ratio of 1:1 to receive either HD201 or referent trastuzumab (loading dose: 8 mg/kg; maintenance dose: 6 mg/kg) every 3 weeks, administered concurrently with 8 cycles of chemotherapy (4 cycles of docetaxel [75 mg/m2], followed by 4 cycles of epirubicin [75 mg/m2]/cyclophosphamide [500 mg/m2]) in the neoadjuvant setting. After surgery, patients received an additional 10 cycles of HD201 or referent trastuzumab in the adjuvant setting according to the previous allocation.
Outcomes
Secondary objectives included evaluation of event-free survival (EFS) (defined as the time from randomization to the first observation of disease progression, including local and distant recurrence, second primary cancer, or death due to any cause), overall survival (OS) (defined as the time from randomization to death), safety, and immunogenicity. Exploratory analyses were conducted for EFS including locally assessed tpCR and bpCR as covariates.
Statistical analysis
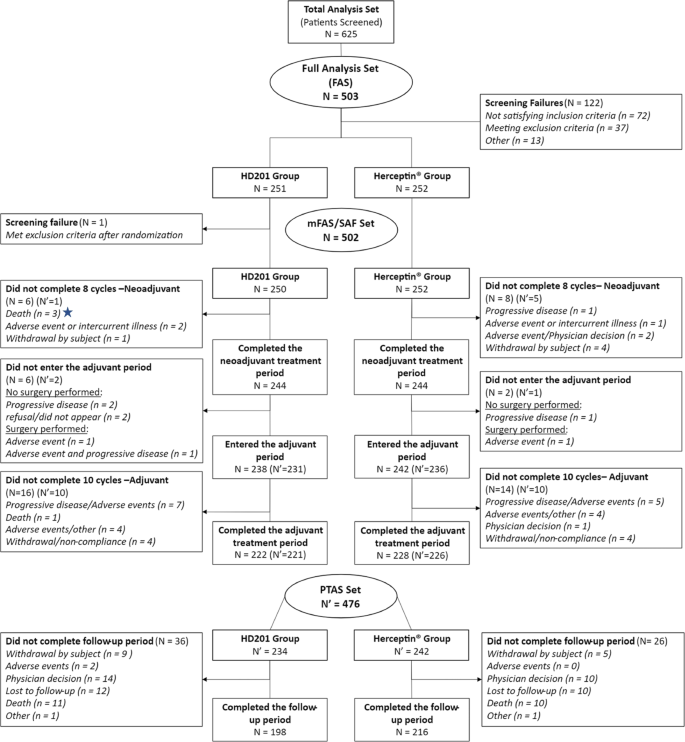
Target sample sizes and statistical power calculations for the primary analysis have been reported previously [1]. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., NC, USA). Kaplan–Meier analysis was used to estimate EFS and OS rates. Cox proportional hazards regression analyses providing hazard ratios (HRs) and 95% confidence intervals (95% CIs) for EFS adjusted for region, stage, and tumor hormonal receptor status are presented. Survival analyses were conducted in the per-protocol set (PPS), including all patients who received the study treatment (without a major protocol deviation affecting the primary efficacy assessment) and who underwent surgery after the completion of neoadjuvant treatment or did not undergo surgery because of lack of efficacy, and analysis was also performed in the modified full analysis set (mFAS), including all patients who received at least 1 dose of study medication (Fig. 1). Safety analyses were descriptive and conducted in all patients who received at least one dose of treatment. Adverse events (AEs) and serious AEs (SAEs) were recorded and graded per standard common technology criteria for adverse events (CTCAE).
Results
Patient population
This analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). The mFAS comprised 502 randomized and treated patients, among whom 250 (49.8%) were in the HD201 group and 252 (50.2%) were in the referent trastuzumab group and were included between February 19 and September 21, 2018, across 70 centers in 12 countries. A total of 28 patients with mFAS were excluded from the PPS (12 patients in the HD201 treatment group and 16 patients in the referent trastuzumab group). The PPS thus comprised 238 patients in the HD201 treatment group and 236 patients in the referent trastuzumab treatment group. Baseline demographics and disease characteristics were well balanced between the study arms as reported previously [1].
Efficacy
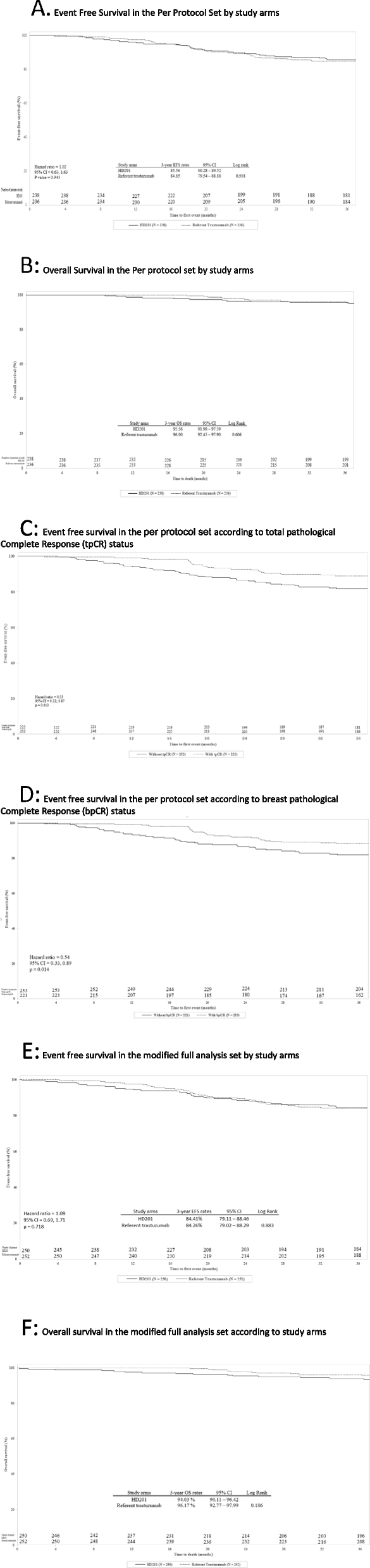
In the PPS, the 3-year EFS rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (Fig. 2A). The Cox proportional HR adjusted for region, stage, and tumor hormonal receptor status was 1.02 (95% CI: 0.63–1.63; p = 0.945) (Fig. 2A). The 3-year OS rates were comparable for the HD201 (95.5%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606) (Fig. 2B). These results for EFS and OS were similar to those in the mFAS population (Figs. 2E and F). The sensitivity analysis searching heterogeneity of treatment effect according to the disease characteristics did not observed any discordances between the two arms in terms of survival outcomes.
Event-Free Survival and Overall Survival in the Per Protocol set (PPS) and in the modified Full Analysis set (mFAS). A EFS by study arm in the PPS. B OS by study arm in the PPS. C EFS by tpCR status in the PPS. D EFS by bpCR status in the PPS. E Event free survival in the mFAS. F Overall survival in the mFAS. bpCR, breast pathologic complete response; CI, confidence interval; EFS, event-free survival; HR, hazard ratio; PPS, per protocol set; OS, overall survival; pCR, pathologic complete response; tpCR, total pathologic complete response
Locally assessed pCR and long-term efficacy
In the PPS, in both treatment arms, 3-year EFS was more better for patients achieving a tpCR (locally assessed) than for those with residual disease, with 10.8% (24/222) versus 17.9% (45/252) of patients with events counting for EFS, respectively (HR 0.53, 95% CI 0.32–0.87; p = 0.013) (Fig. 2C). Similarly, 3-year EFS was more favorable for patients achieving a bpCR (locally assessed) than for those without (HR 0.54, 95% CI 0.33–0.89; p = 0.014) (Fig. 2D).
Long-term safety
During the posttreatment follow-up period, PTAEs were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the referent trastuzumab groups, respectively (Table 1). PTAEs with severity grade 3 or higher were reported for 7 (3.0%) patients and 13 (5.4%) patients, and serious PTAEs were reported for 4 (1.7%) patients and 5 (2.1%) patients, respectively. No serious PTAEs related to study treatment were reported during the posttreatment follow-up period. Overall, no noteworthy differences were found between the two groups.
Discussion
The phase 3 TROIKA study in patients with ERBB2-positive EBC is the conclusive step in the investigation of HD201 and the referent trastuzumab in the extensive comparison of the two supporting the development of the biosimilar candidate [1]. Analysis of the secondary long-term efficacy endpoints, EFS and OS, after 3 years of follow-up continues to support the equivalence of HD201 to referent trastuzumab established by the primary analysis based on the tpCR criterion. Most recurrent events in ERBB2-positive breast cancer have been reported to occur within 3 years, and this duration appears sufficient to provide adequate evidence to support efficacy and safety conclusions [12,13,14]. Achieving tpCR was associated with longer EFS in both treatment arms, and these results were consistent with those observed in other studies assessing neoadjuvant trastuzumab [9,10,11, 14].
The overall safety profile of HD201 and trastuzumab at the 3-year follow-up remains consistent with the safety profiles observed in previous studies, post-treatment adverse events are unrelated or unlikely to the study drug, and rarely, events related to the study drug occurred in the post-treatment follow-up period.
Limitations of the study include the use of newer anti-HER2 agents, which could impact survival in patients with relapse and were not assessed in this study. In addition, subgroup analyses are limited by their small and unbalanced sample sizes.
Conclusions
This final analysis of TROIKA further supports the comparability of the efficacy and safety of HD201 and the referent trastuzumab.
Availability of data and materials
Data types: Deidentified participant data.
How to access data: The application providing the project details should be submitted to Prestige Bio Pharma,(2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118,222) or by email at jamie.kim@prestigebio.com or x.pivot@icans.eu. Then the request need to be approved by the steering committee of the study before release the data.
Restriction: The steering committee of the trial approval based on the scientific assessment of the application is requested to release the data.
References
Pivot X, Georgievich MA, Shamrai V, Dzagnidze G, Soo Hoo HF, Kaewkangsadan V, Petrelli F, Villanueva C, Nikolaevich LO, Hii J, et al. Efficacy of HD201 vs referent trastuzumab in patients with erbb2-positive breast cancer treated in the neoadjuvant setting: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2022;8(5):698–705.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England). 2014;384(9938):164–72.
Stebbing J, Baranau Y, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, Zhavrid E, Boliukh D, Stroyakovskii D, Pikiel J, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18(7):917–28.
von Minckwitz G, Colleoni M, Kolberg HC, Morales S, Santi P, Tomasevic Z, Zhang N, Hanes V. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987–98.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, Pienkowski T, Lichinitser M, Semiglazov V, Melichar B, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869–78.
Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, Vinnyk Y, Im SA, Sarosiek T, Chatterjee S, et al. Phase iii, Randomized, double-blind study comparing the efficacy, safety, and immunogenicity of sb3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2018;36(10):968–74.
Pivot X, Cox DG. A new era for treatment development in HER2-positive breast cancer. Lancet Oncol. 2018;19(2):160–2.
Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, Im SA, Sarosiek T, Chatterjee S, Wojtukiewicz MZ, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: Final safety, immunogenicity and survival results. Eur J Cancer. 2018;93:19–27.
Jackisch C, Stroyakovskiy D, Pivot X, Ahn JS, Melichar B, Chen SC, Meyenberg C, Al-Sakaff N, Heinzmann D, Hegg R. Subcutaneous vs Intravenous Trastuzumab for Patients With ERBB2-Positive Early Breast Cancer: Final Analysis of the HannaH Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019;5(5):1–5. https://doi.org/10.1001/jamaoncol.2019.0339.
Stebbing J, Baranau YV, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, Zhavrid E, Boliukh D, Pikiel J, Eniu AE, et al. Long-term efficacy and safety of CT-P6 versus trastuzumab in patients with HER2-positive early breast cancer: final results from a randomized phase III trial. Breast Cancer Res Treat. 2021;188(3):631–40.
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet (London, England). 2019;393(10191):2591–8.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377(2):122–31.
Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, Moliterni A, Vazquez F, Byakhov MJ, Lichinitser M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7.
Acknowledgements
Nothing
Funding
Funding/Support: This study was sponsored by Prestige Biopharma Ltd.
Role of the Funder/Sponsor: The funding source validated the study as designed by the trial’s steering committee, as well as subsequent amendments. The sponsor organized the management and the conduction of the study, including the collection of data. The data were analyzed by DICE with Drs Derde and Kaufman. The data were interpreted by the trial’s steering committee, including Drs. Pivot, Cox, Deforce, Feyaerts, and Derde, independently from the sponsor. The preparation of the manuscript was performed by Drs. Pivot and Derde and reviewed and approved by all enclosed authors. The sponsor approved the manuscript and agreed to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Dr. Pivot had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Pivot, Kaufman. Acquisition, analysis, or interpretation of data: Pivot, Dzagnidze, Georgeivich, Shamrai, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Drafting of the manuscript: Pivot, Derde. Critical revision of the manuscript for important intellectual content: Pivot, Georgeivich, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Statistical analysis: Pivot, Kaufman, Derde, Deforce, Cox. Administrative, technical, or material support: Kim, Pradhan, Jaison, Feyaerts, Supervision: Pivot, Feyaerts. All author read and accept the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
TROIKA trial (NCT03013504) that was reported according to the Enhancing the Quality and Transparency Of Health Research guidelines. The TROIKA trial was conducted according to the ethical principles of good clinical practice and was approved by ethics committees in each involved country. All patients signed an informed consent to participate in the trial which are available at request submitted to Prestige Bio Pharma, (2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118222). An independent monitoring committee monitored the study.
List of the ethics committees / institutional review board that approved the study.
Country | Site Code | IEC | Address |
|---|---|---|---|
Malaysia | 458–001 | University of Malaya Medical Centre Medical Research Ethics | Pusat Perubatan Universiti Malaya Lembah Pantai 59100 Kuala Lumpur, Malaysia |
458–002 | Research Ethics Committee; Universiti Kebangsaan Malaysia | ||
458–003 | Medical Research and Ethics Committee, | National Institutes of Health, Ministry of Health Malaysia, Block A, Level 2, No 1, Jalan Setia Murni U13/52, Seksyen U13, Setia Alam, 40170, Shah Alam, Selangor | |
458–005 | Human Research Ethics Committee of Universiti Sains Malaysia | Human Research Ethics Committee USM, Division of Research & Innovation (R&I), USM Health Campus, 16150, Kubang Kerian, Kelantan | |
458–006 | Medical Research and Ethics Committee, | National Institutes of Health, Ministry of Health Malaysia, Block A, Level 2, No 1, Jalan Setia Murni U13/52, Seksyen U13, Setia Alam, 40170, Shah Alam, Selangor | |
458–008 | Medical Research and Ethics Committee, | National Institutes of Health, Ministry of Health Malaysia, Block A, Level 2, No 1, Jalan Setia Murni U13/52, Seksyen U13, Setia Alam, 40170, Shah Alam, Selangor | |
458–009 | Medical Research and Ethics Committee, | National Institutes of Health, Ministry of Health Malaysia, Block A, Level 2, No 1, Jalan Setia Murni U13/52, Seksyen U13, Setia Alam, 40170, Shah Alam, Selangor |
Country | Site Code | Type IEC | IEC | Address |
|---|---|---|---|---|
Bulgaria | 100–005 | Central | MINISTRY OF HEALTH ETHICS COMMITTEE FOR CLINICAL TRIALS | 8, Damyan Gruev Str., 1303, Sofia, |
Estonia | 233–001 | Central | TMREC: Tallinn Medical Research Ethics Committee | Hiiu 42, Tallinn 11619, Estonia |
France | 250–006 | Central | CPP EST IV – Hôpital Civil | 1, place de l'Hôpital 67091 STRASBOURG Cedex |
Italy | 380–002 | Local | Comitato Etico dell’ Area Vasta Emilia Nord | Via del Pozzo, 71 – 41124 Modena |
380–005 | Local | Comitato Etico dellÁrea Vasta Emilia Nord | Via G. Taberna, 49- Edificio 7 – Ingresso B, piano rialzato, 29121 Piacenza | |
380–006 | Local | Comitato Etico per le sperimentazioni cliniche della provincia de Vicenza | ||
380–007 | Local | COMITATO ETICO REGIONE TOSCANA | AREA VASTA NORD OVEST Via Roma, 67 | |
380–008 | Local | Comitato Etico Regione Toscana | Area Vasta Sud Est Via Senese 161, 58100 Grosseto | |
380–010 | Local | COMITATO ETICO DELLA ROMAGNA CEROM VIA PIERO MARONCELLI, 40 | ||
380–010 | Local | IRST Scientific Medical Committee | ||
380–010 | Local | Comitato etico, Regione Toscana | Area Vasta Sud Est | |
380–010 | Local | COMITATO ETICO DELLA ROMAGNA | CEROM VIA PIERO MARONCELLI, 40 | |
380–013 | Local | Comitato Etico Bergamo Piazza OMS | Organizzazione mondiale della sanita, 1 | |
380–015 | Local | Comitato Etico IRCCS Di Candiolo Strada Provinciale 142 | ||
380–004 | Central | 00144 ROMA | via Chianesi, 53 | |
380–004 | Central | 143 ROMA | via Chianesi, 53 | |
380–005 | Local | Comitato Etico dellÁrea Vasta Emilia Nord | Via G. Taberna, 49- Edificio 7 – Ingresso B, piano rialzato, 29121 Piacenza | |
380–006 | Local | Comitato Etico per le sperimentazioni cliniche della provincia de Vicenza | ||
380–002 | Local | Comitato Etico dell’ Area Vasta Emilia Nord | Via del Pozzo, 71 – 41124 Modena | |
380–013 | Local | Comitato Etico Bergamo Piazza OMS | Organizzazione mondiale della sanita, 1 | |
380–015 | Local | Comitato Etico IRCCS Di Candiolo | Strada Provinciale 142 | |
Poland | 616–001 | Central | Komisja Bioetyezna przy Okregowej Lekarskiej w Lublinie | |
616–002 | Central | Komisja Bioetyezna przy Okregowej Lekarskiej w Lublinie | ||
Spain | 724–003 | Central | Ethics Committee for Drug Research (CEIm) Regional of the Community of Madrid | C/ Customs, 29—3rd Floor 28013 Madrid |
724–001 | Central | Research Ethics Committee Center of the Unversity Hospitals Virgen Macarena—Virgen del Rocio de Sevilla | ||
724–004 | Central | Autonomous Ethical Committee for Clinical Studies of Medicines and Health Products of the Valencian Community (CAEC) |
COUNTRY | NAME of the RA | RA ADDRESS |
|---|---|---|
Bulgaria | Bulgaria Drug Agency | 8, Damyan Gruev Str., 1303 Sofia, Bulgaria |
Estonia | RAVIVIAMET State Agency of Medicines | Nooruse 1, 50411 Tartu |
France | ANSM | 143/147, bd Anatole France, 93285 Saint Denis cedex Paris, |
Italy | AIFA | Via del Tritone, 181—00187 Roma |
Poland | PREZES Office for Registration of Medicinal Products, Medical Devices and Biocidal Products | Ul. Zabkowska 41, 03–736 Warszawa |
Spain | AEMPS | Calle Campezo, 1, 28022 Madrid |
Bulgaria | Bulgaria Drug Agency | 8, Damyan Gruev Str., 1303 Sofia, Bulgaria |
Country | Site Code | IEC |
|---|---|---|
Belarus | 112–001 | Ethics Committee of Minsk city clinical oncological dispensary,64 Nezavisimosti Ave., Minsk, 22013 |
112–002 | Ethics Committee of Vitebsk Regional Oncological Dispensary P.Brovki str., 33, Vitebsk, 210603 | |
112–003 | Ethics Committee of Mogilov Regional Oncological Dispensary, Academic Pavlova str., 2a, Mogilov, 212018 | |
112–004 | Ethics Committee of Brest Regional Clinical Oncological Dispensary, Meditskinskaya str., Brest 224027 | |
112–005 | Ethics Committee of N.N.Alexandrov national cancer center of Belarus, s. Lesnoy-2, Minsk, 223040 | |
Georgia | 268–001 | Independent Ethics committee of "Unimedi Ajara” Ltd |
268–002 | Ethical CommitteeS. Khechinashvili University Hospital | |
268–003 | Independent Ethics committee of "Unimedi AjaraOncology center", new name Independent Ethics committee of Evex Medical Corporation " oncology center (from 03 December 2018), new name Independent Ethics committee of "Evex Hospitals" oncology center (from May 2019) | |
268–004 | Ethics committee of Cancer center of Adjara Autonomous Republic LTD, new name Ethics committee of LTD “High Technology Hospital Medcenter (from 16 May 2018) | |
268–005 | Independent Ethics committee of ST NICHOLAS CENTER FOR SURGERY and ONCOLOGY" Ltd new name Independent Ethics committee JSC EVEX Medical Center (from 03 December 2018), from May 2019 new name Independent Ethics committee JSC EVEX Clinic | |
268–006 | Ethics committee of Research Institute of Clinical Medicine” Ltd | |
268–007 | Independent Ethics committee of Institute of Clinical Oncology " LTD | |
268–008 | Independent Ethics committee of Multiprofile Clinic Consilium Medulla" | |
268–009 | Independent Ehics Committee of Cancer Research Center” Ltd | |
268–010 | Independent Ehics Committee of Tbilisi Cancer Center Ltd | |
Russia | 643–001 | Local Ethics Committee of State Autonomous Healthcare Institution Republic Clinical Oncology Dispensary of the Ministry of Health of Republic of Tatarstan |
643–002 | Independent Ethics Committee of State Budgetary Healthcare Institution Tambov Regional Oncology Clinical Dispensary | |
643–003 | Local Ethics Committee of State Bugetary Healthcare institution "Leningrad Regional Oncology Dispensary", new name Local Ethics Committee State Bugetary Healthcare institution "Leningrad Regional Oncology Dispensary named after L.D. Roman" | |
643–004 | Local Ethics Committee of State Bugetary Healthcare institution "Leningrad Regional Oncology Dispensary", new name Local Ethics Committee State Bugetary Healthcare institution "Leningrad Regional Oncology Dispensary named after L.D. Roman" | |
643–005 | Ethica committe at "Republican Clinical Oncology Dispensary of Ministry of Health of Bashkortostan Republic" | |
643–006 | Ethics Committee of Moscow State Budgetary Healthcare Institution Moscow City Oncologic Hospital No. 62 of Moscow Healthcare Department. From 10/04/2019 Independent Inetrdisciplinary committee on Ethica Review of Clinical studies | |
643–007 | Local Ethics Committee of State Budgetary Healthcare Institution Orenburg Regional Clinical Oncologic Dispensary | |
643–008 | Local Ethics Committee of Ryazan State Medical University n.a. academician I.P.Pavlov" of the Ministry of Health of the Russian Federation | |
643–009 | Ethics Committee at State Budgetary Healthcare Institution of Ryazan Region Regional Clinical Oncology Dispensary | |
643–010 | Ethica committee at Budgetary Healthcare Institution of Omsk Region Clinical Oncologic Dispensary | |
643–011 | Ethics Committee at Saint Petersburg City Clinical Oncologic Dispensary | |
643–012 | Ethical Committee of Regional budgetary Healthcare institution Kursk Regional clinical oncology dispensary | |
643–013 | Ethics Committee of Limited Liability Company EVIMED | |
643–014 | Independent Ethics committee of MEDSI | |
643–017 | Local Ethics Committee of FGBOU VO North-Western State Medical University named after I.I. Mechnikov of the Ministry of Health of the Russian Federation | |
643–018 | The Ethics Committee of OOO Komanda | |
643–019 | The Local Ethics Committee of State Budgetary Healthcare Institution of Stavropol Region Pyatigorsk Interdistrict Oncologic Dispensary | |
643–021 | Ethics Committee of Limited Liability Company VitaMed | |
643–022 | Federal State Budgetary Institution National Medical Research Center of Oncology named after N.N. Petrov of the Ministry of Health of the Russian Federation | |
643–023 | Independent Ethics committee of MEDSI | |
643–024 | Independent Interdisciplinary Committee on Ethics Review of Clinical Studies | |
804–001 | Committee on Ethics at the MI “Dnipropetrovsk City multiprofile Clinical Hospital #4” of Dnipropetrovsk Regional Council* | |
804–002 | Committee on Bioethics and Deontology of SI “Zaytsev V.T. Institute of General and Urgent Surgery of NAMS of Ukraine” | |
804–003 | Committee on Ethics at the Zaporizhzhya Regional Clinical Oncology Dispensary of Zaporizhzhya Regional Council | |
Ukraine | 804–004 | Local Ethics Committee at “Lviv State Regional Oncology Treatment and Diagnostic Center” |
804–005 | The Committee on Ethics at the “Volyn Regional Oncological Dispensary” | |
804–006 | The Committee on Ethics at the Central City Clinical Hospital of the City of Uzhgorod | |
804–007 | The Committee on Ethics at Podillya Regional Oncology Center | |
804–008 | The Committee on Ethics at MI KRC Kyiv regional oncology dispensary" |
COUNTRY | NAME of the RA |
|---|---|
Belarus | Ministry of Health of the Republic of Belarus |
Georgia | State Regulatory Agency for Medical Activities of Ministry of labour, Health and Social Affairs of Georgia |
Russia | Ministry of Health of Russian Federation |
Ukraine | State Expert Center of Ministry of Health of Ukraine |
COUNTRY | NAME of the RA | RA ADDRESS |
|---|---|---|
Thailand | Food and Drug Administration Thailand, Ministry of Public Health | 88/24 Tiwanon Road Nonthaburi, Thailand 11000 |
Country | Site Code | Type IEC | IEC | Address |
|---|---|---|---|---|
THAILAND | 764–001 | IRB | Institutional Review Board Faculty of Medicine Siriaj Hospital | His Majesty the King's 80th Birthday Anniversary 5th December 2007, Building 2nd Floor Room 2102 Wang Lang Road Bangkoknoi, Bangkok 10700 |
764–002 | IRB | Center for Ethics in Human Research, Khon Kaen University | 17th Floor Somdej Phra Srinakarinda Boromratchachoonnani Memorial Building (Sor Wor. 1) Faculty of Medicine Khon Kaen University | |
764–004 | IRB | Ethics Committee, National Cancer Institute | The IRB, Royal Thai Army Medical Department 317/5 Rajavithi Road, Rajathevee, Bangkok 10400, Thailand |
COUNTRY | NAME of the RA | RA ADDRESS |
|---|---|---|
Malaysia | National Pharmaceutical Regulatory Agency (NPRA) | 36, Jln Professor Diraja Ungku Aziz, Pjs 13, 46200 Petaling Jaya Selangor, Malaysia |
Consent for publication
Not applicable.
Competing interests
Conflict of Interest Disclosures: Dr. Pivot reported being an unpaid adviser for Prestige Biopharma. Dr Dzagnidze reported personal fees from Khechinashvili University Hospital during the conduct of the study. Dr Kaewkangsadan reported grants from Prestige BioPharma during the conduct of the study. Drs Derde, Kaufman, and Deforce are/were employees of DICE Ltd. and had a memorandum of understanding with Prestige BioPharma Ltd. No other disclosures were reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pivot, X., Manikhas, A.G., Shamrai, V. et al. Final analysis of the phase 3 randomized clinical trial comparing HD201 vs. referent trastuzumab in patients with ERBB2-positive breast cancer treated in the neoadjuvant setting. BMC Cancer 23, 112 (2023). https://doi.org/10.1186/s12885-023-10574-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10574-2