- Research
- Open access
- Published:
Population-based BRCA germline mutation screening in the Han Chinese identifies individuals at risk of BRCA mutation-related cancer: experience from a clinical diagnostic center from greater Shanghai area
BMC Cancer volume 24, Article number: 411 (2024)
Abstract
Background
Deleterious BRCA1/2 (BRCA) mutation raises the risk for BRCA mutation-related malignancies, including breast, ovarian, prostate, and pancreatic cancer. Germline variation of BRCA exhibits substantial ethnical diversity. However, there is limited research on the Chinese Han population, constraining the development of strategies for BRCA mutation screening in this large ethnic group.
Methods
We profile the BRCA mutational spectrum, including single nucleotide variation, insertion/deletion, and large genomic rearrangements in 2,080 apparently healthy Chinese Han individuals and 522 patients with BRCA mutation-related cancer, to determine the BRCA genetic background of the Chinese Han population, especially of the East Han. Incident cancer events were monitored in 1,005 participants from the healthy group, comprising 11 BRCA pathogenic/likely pathogenic (PLP) variant carriers and 994 PLP-free individuals, including 3 LGR carriers.
Results
Healthy Chinese Han individuals demonstrated a distinct BRCA mutational spectrum compared to cancer patients, with a 0.53% (1 in 189) prevalence of pathogenic/likely pathogenic (PLP) variant, alongside a 3 in 2,080 occurrence of LGR. BRCA1 c. 5470_5477del demonstrated high prevalence (0.44%) in the North Han Chinese and penetrance for breast cancer. None of the 3 LGR carriers developed cancer during the follow-up. We calculated a relative risk of 135.55 (95% CI 25.07 to 732.88) for the development of BRCA mutation-related cancers in the BRCA PLP variant carriers (mean age 42.91 years, median follow-up 10 months) compared to PLP-free individuals (mean age 48.47 years, median follow-up 16 months).
Conclusion
The unique BRCA mutational profile in the Chinese Han highlights the potential for standardized population-based BRCA variant screening to enhance BRCA mutation-related cancer prevention and treatment.
Key message of article
There is significant ethnical diversity in the prevalence and spectrum of BRCA germline variants. While previous studies of regional and preliminary national BRCA mutation screening have contributed to our knowledge of BRCA germline mutation in China, our research has unveiled a distinctive mutational profile in the Han Chinese across major regions of the country, representing 20% of the world’s population. It also demonstrated the potential of BRCA mutation screening in the general healthy population for identifying individuals at higher risk of BRCA mutation-related cancer, a risk often overlooked by family history-based screening strategies. These findings offer initial insights into the potential benefits of population-based screening for preventing BRCA mutation-related cancer in the Chinese Han. Further investigation is warranted, including multi-center, long-term prospective trials, cost-effectiveness analysis, and psychosomatic medical research.
Introduction
Deleterious germline variants of BReast CAncer susceptibility genes BRCA1 and BRCA2 (BRCA) significantly increase the risk of developing “BRCA mutation”-related tumors, including breast, ovarian, pancreatic, and prostate cancer [1]. Screening for these variants in those with a family cancer history has enhanced the early prevention and intervention among high-risk individuals [2, 3].
Large-scale genome databases have expanded our understanding of BRCA’s genetic background in the major populations [4], highlighting ethnic diversity in both the prevalence and mutational spectrum of germline BRCA variation across Caucasians, Ashkenazi Jews, Hispanics, African Americans, and Asian [5, 6]. In addition, it has brought to light the surprising observation that population-based screening can identify nearly twice as many deleterious variant carriers compared to conventional family history-based screening [7, 8].
In the last five years, there have been over 40 published studies profiling the mutational spectrum in patients with BRCA mutation-related cancers in China [9,10,11,12,13]. While some of them revealed the mutational landscape in the healthy controls of case-control studies for breast cancer [9, 14] and ovarian cancer [10], investigations into the prevalence of mutations in the major population (Chinese Han) and the subsequent research on whether variant screening can yield benefits remains limited due to the extensive geographical landscape of China and the significant genetic diversity within the Han ethnic group [15]. Regional studies have documented varying prevalence of single nucleotide variations (SNVs) and small insertion and deletion events (InDels) in areas like Taiwan [16] and Macau [17]. There was also nationwide variant screening conducted, but the participants predominately originates from the North Han and Lingnan Han [18]. Besides, large genomic rearrangements (LGRs), another contributor to the silence of BRCA function, have been less reported in this population. It remains uncertain whether broadening BRCA screening in this demographic offers more benefits in identifying high-risk individuals [19, 20]. These gaps in our knowledge of BRCA variants’ genomic and functional aspects have impeded the establishment and standardization of BRCA mutation screening strategy for the Chinese Han population, which constitutes over 20% of the global population.
In this descriptive study, we integrated next-generation sequencing (NGS) data of BRCA1 and BRCA2 exons from 2,080 apparently healthy individuals and 522 patients with BRCA mutation-related cancer, to reveal the unique genetic pattern of deleterious BRCA variants, including SNVs, InDels, and LGRs, in the general Chinese Han population, with a special focus in the East Han, which account for 25% population of the Chinese Han population. Additionally, with clinical follow-up data spanning up to 24 months in the healthy population, we demonstrate that BRCA germline mutation screening can aid in the risk stratification and early detection of BRCA mutation-related cancer in the apparently healthy Chinese Han population.
Participants and methods
Apparently healthy population and patients with BRCA mutation related-cancer
From June 2021 to February 2023, 2,080 apparently healthy participants who denied either a personal or family history of cancer were enrolled from the health management center of Huashan Hospital, Fudan University. All the participants were over 18 years old, and their medical records were blindly reviewed by two physicians to confirm the tumor-free status at enrollment. Besides, 121 patients with triple-negative breast cancer (TNBC), 181 with metastatic castration-resistant prostate cancer (mCRPC), 215 with pancreatic ductal adenocarcinoma (PDAC), and 5 with high-grade ovarian cancer (HGOC) seen in Huashan Hospital, Fudan University were enrolled as the BRCA mutation related-cancer group. All the cancer patients were enrolled to undergo BRCA mutation screening with the aim of formulating surgery and chemo-/radio-therapy strategies guided by their genotypes [21]. The cancer diagnosis were established based on blind review of biopsy or mastectomy slides by 2 certificated pathologists, in accordance to the World Health Organization tumour classification blue book [22,23,24,25]. The Han ethnicity and place of birth were confirmed in the electronic healthcare registration system. Written informed consent was received from all participants. In compliance with the Helsinki Declaration of 1975, as revised in 1996, this study was approved by the Institutional Review Board of Huashan Hospital of Fudan University (2023 − 812).
Germline mutation profiling of BRCA1 and BRCA2 by next generation sequencing
Genomic DNA was extracted from ethylenediaminetetraacetic acid anti-coagulated blood using the QIAamp DNA blood mini kit (Qiagen, #51,104). Sequencing library was construction with the BRCA1 and BRCA2 gene mutation detection V2 kit (Amoy Diagnostics, #8.06.0092) and sequenced using the MiSeqDx system (Illumina Inc, CA) with a minimum coverage of 200×, uniformity of 95%, and Q30 for over 85% bases.
The germline mutation was called and filtered using the commercial software SSBC-VarScanv1.1.0 developed by Amoy Diagnostics (Xiamen, China). All candidate SNVs or InDels were hard filtered and further confirmed in Integrative Genomics Viewer (IGV). The germline variants in BRCA1 (MANE NM_007294.4) and BRCA2 (MANE NM_000059.4) were classified into five categories, including benign, likely benign, variants of uncertain significance, likely pathogenic, and pathogenic following the American College of Medical Genetics (ACMG) guideline (for detailed variant classification protocol, refer to Supplementary File 1 and 2) [26]. BRCA databases, including BIC, ClinVar, BRCA Exchange, and LOVD3.0 were used for the population comparative analysis.
Detection of large genomic rearrangements and confirmation by multiplex ligation probe amplification (MLPA)
The germline copy number variation (CNV) was identified by the AmpliconCnvCaller software from Amoy Diagnostics. Samples with significant CNV in two or more regions of one gene were considered as candidates harboring BRCA LGRs and subjected to SALSA MLPA assays (MRC Holland, #P002 for BRCA1 and #P090 for BRCA2) on a PRISM 3500 DNA analyzer (Applied Biosystems, MA) and further validated by the independent kits (MRC Holland, #P087 for BRCA1 and #P077 for BRCA2).
Follow-up of the apparently healthy participants
The apparently healthy participants received detailed BRCA mutation test results through post-test counseling. Those with BRCA pathogenic/likely pathogenic (PLP) variants received guidance from the clinical oncologist on self-examination and health follow-ups. From June 2021 to June 2023, 1,005 out of the 2,080 healthy individuals visited to the health management center every 6 to 14 months for tumor risk screening, which included mammography/MRI, breast physical examination (for breast cancer risk), transvaginal ultrasound and CA125 (for ovarian cancer risk), abdominal CT/MRI, CA199 (for pancreatic cancer risk, imaging test was only performed in the individuals with PLP variant), and digital rectal examination, prostate-specific antigen (PSA) (for prostate cancer risk). Over the 24 months during project period, 412 individuals underwent one examination, 375 individuals underwent two, and 218 individuals underwent three follow-ups.
Association for clinical genomic science (ACGS) classification and computational scoring of variants of unknown significance (VUS)
The VUS obtained by the ACMG criteria were further classified into six categories of pathogenicity: hot, warm, tepid, cool, cold, and ice cold, according to the ACGS classification guideline [27]. Given that the P/LP variants in BRCA have emerged in recent human history, rather than deriving from non-human species [28], the evolution conservation-based function prediction tools such as SIFT and polyphen2, were not suitable for annotating missense VUS [29]. Accordingly, these VUS were analyzed for functional pathogenicity with the predictive scoring data from the DNA/protein sequence machine learning-based software iMutant [30], MutaionTaster [31], VEST [32], EVE [33] and REVEL [34].
Statistical analysis
Statistical analysis and data visualization was performed with R (v4.0.2). Comparison of continuous values was performed using a two-sample t-test or Mann-Whitney U test if appropriate. Categorical values were compared with Fisher’s exact test. Statistical significance was defined as a two-sided P < 0.05.
Results
Demographic and genetic background of the participants
The median age for healthy participants was 49.05 (18 to 88) years and 59.77 (19 to 82) years for cancer patients. No gender bias was observed in the healthy group and PDAC patients (Table 1).
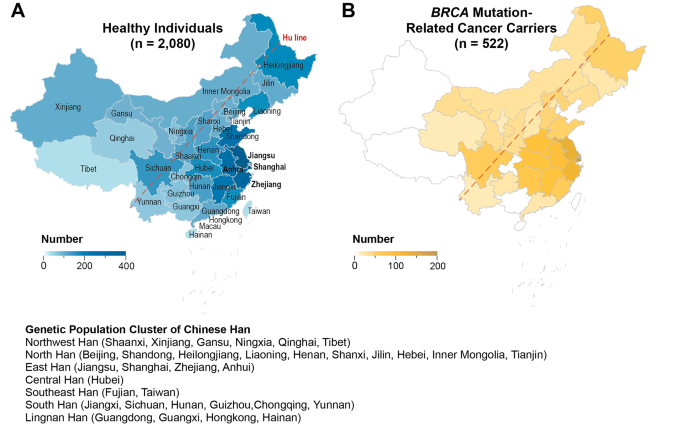
Among all the 2,080 apparently healthy individuals, there were ten pairs of self-reported first-degree relatives. Three self-reported first-degree relatives were enrolled among all the 522 cancer carriers. The geographic constitution for both the healthy group and cancer cohorts was illustrated in Fig. 1, with the healthy individuals from 33 out of all 34 administrative regions of China, except for Macau Special Administrative Region (SAR), and cancer patients from 25 of these regions. Most of the study population was from the Greater Shanghai area (for healthy group, 17.02% from Shanghai Municipality, 18.7% from Jiangsu Province, 12.99% from Zhejiang Province; for tumor patients, 34.99% from Shanghai Municipality, 21.03% from Jiangsu Province, 14.72% from Zhejiang Province). 97.63% (1960/2020) of the enrolled healthy individuals and 98.45% (509/517) cancer patients are from the region east of the Hu-line, which covered 93% of the population of China. According to the report from ChinaMAP [15], we also subdivided the participants into seven distinguished population clusters, including Northwest Han, North Han, East Han, Central Han, Southeast Han, South Han, and Lingnan Han (Fig. 1). In short, the top three large subpopulation of this study are the East Han (56.40% of healthy individuals and 77.20% of cancer patients), North Han (21.92% of healthy individuals and 9.77% of cancer patients), and South Han (10.14% of healthy individuals and 6.51% of cancer patients). The detailed composition of participants was listed in Supplementary File 3.
Geographic distribution of the 2,080 healthy individuals and 522 patients with BRCA mutation-related cancer. (A) Birthplace of the healthy individuals across 33 out the 34 provinces, municipalities and autonomous regions in China (except for Macau SAR), with the majority from east coast and central region (48.71%). (B) The major cancer patients are from eastern region of China, represented by the Greater Shanghai area (70.74%)
BRCA germline variations in the general Chinese Han population and BRCA mutation related-cancer cohorts
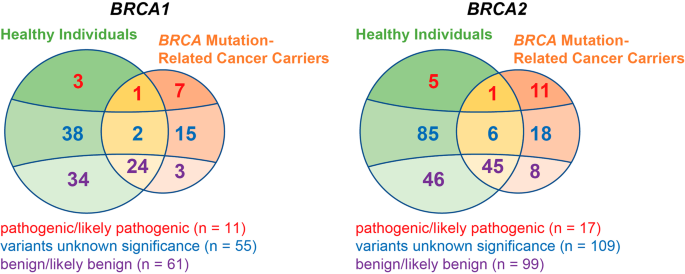
We gathered 352 distinct germline variants (127 for BRCA1 and 225 for BRCA2) from 2,080 Han Chinese healthy individuals and 522 patients with BRCA mutation-related cancer. Among these variations, 211 were specifically identified in healthy individuals and 62 in cancer patients, while 79 variants were present in both two groups (Fig. 2). Over a quarter (134 out of 352) of the variants were recurrent (carriers ≥ 2). Among them, 4 were PLPs, 29 were VUS, and 101 were benign/likely benign (BLB) variants, with 51 healthy cohort-specific and 4 cancer cohort specific variations.
On average, one healthy individual carried 12.09 BRCA variants (BRCA1: 4.80, BRCA2: 7.29), and one cancer patient harbored 12.20 variants (BRCA1: 4.97, BRCA2: 7.23). No significant difference was observed in the variant burden between the healthy and cancer groups, either for BRCA1 (P = 0.36) or BRCA2 (P = 0.52). Among the healthy population, there was no statistically significant differences in the variant burden among different genders (P = 0.72) and among different age groups (P = 0.85). There was also no significant regional or cancer species aggregation (P = 0.65) of high variation burden. This homogeneity of BRCA variant burden across different demographic and pathogenic factors demonstrated a uniform and stable baseline for BRCA germline variations in the Chinese Han population.
BRCA1/2 pathogenic/likely pathogenic SNV and InDels
Ten PLP variants were identified in the apparently healthy individuals (Table 2) and 20 in the cancer patients (Table 3). There is a 0.53% (11/2080) chance for an individual to harbor the BRCA germline PLP variants within our Chinese Han cohort. There was no significant difference between genders [0.33% (4/1198) in males and 0.79% (7/882) in females, P = 0.22] and age groups [0.57% (10/1751) for < 60 years old vs. 0.30% (1/329) for ≥ 60 years old, P = 1.00] in the incidence of carrying BRCA PLP variants.
The eight healthy individual-specific PLP variants included one frameshift duplication (BRCA2 c.7409dup), four frameshift deletions (BRCA1 c.869del, BRCA1 c.5521del, BRCA2 c.8650del, BRCA2 c.9753del), and three nonsense variants (BRCA1 c.2934T > G, BRCA2 c.47 C > T, BRCA2 c.3599_3600del). Any of these variants was not observed in the 1000 genome resource or gnomAD, except for the BRCA2 c.3599_3600del, which is incorporated in gnomAD with a frequency of 1.09 × 10− 4 (1/9,197) in East Asian and 5.29 × 10− 5 (3/56,761) in non-Finnish European. Moreover, to our knowledge, the BRCA1 c.869del, c.2934T > G, c.5521del, and BRCA2 c.3523 C > T, c.8650del, c.9753del have not been reported by any general population screening study in China. All these eight variants were reported in the ClinVar, BIC, BRCA Exchange, or LOVD database as pathogenic, demonstrating that conducting germline BRCA mutation screening in the general Chinese Han population can identify the individuals carrying deleterious variants.
Two nonsense variants, specifically BRCA1 c.5470_5477del and BRCA2 c.5682 C > G, were identified in both healthy individuals and cancer patients. Of note, the BRCA1 c.5470_5477del was present in 5 unrelated individuals − 2 healthy individuals and 3 TNBC patients, all hailing from the North China provinces (Shandong, Hebei, Henan). This variant, previously reported as a founder mutation in the Chinese Han breast cancer patients [35], demonstrated a significant North Han enrichment in both the cancer patients [3/51 (North Han) vs. 0/471 (non-North Han), P = 8.84 × 10− 4] and healthy individuals [2/456 (North Han) vs. 0/1624 (non-North Han, P = 0.048). The BRCA2 c.5682 C > G was found in 2 unrelated individuals: 1 healthy person and 1 TNBC, both originating from the East Han (Zhejiang and Shanghai). This mutation has been collected in the gnomAD non-Finnish European population, albeit at a low frequency of 1.77 × 10− 5 (1/56,574), but it was absent in the other gnomAD population or 1000 genome. Heterozygotes made up all bearers of the PLP variants. Additionally, the recurrent BRCA2 c.3847_3848del variant was identified exclusively in PDAC patients from the East (Shanghai and Anhui).This variant has also been reported in previous regional studies in the East and Southeast Han (1/2769 unselected breast cancer patient in Zhejiang [36], 1/316 prostate cancer patient in Shanghai [37], and 1/6,314 normal Macan [17]).
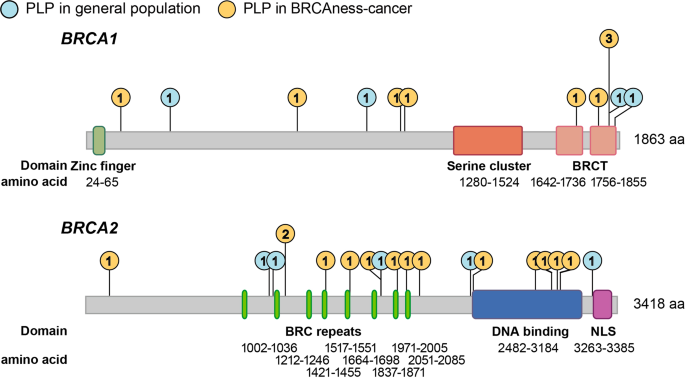
Gene-level analysis of variant prevalence revealed no significant enrichment of PLP variants in specific genes when comparing the healthy individuals (4 for BRCA1, 6 for BRCA2) and cancer patients (8 for BRCA1, 12 for BRCA2) (Fisher’s Exact P = 0.745). The frequency distribution of PLP variants in the gene structures, including UTR, intron, and exon, was similar among healthy individuals and tumor patients for both BRCA1 and BRCA2 (Supplementary File 4). However, there was a significant aggregation of PLP variants in the BRC repeats (P = 0.019) and DNA binding domain (P = 0.015) of BRCA2 in cancer patients, whereas in healthy individuals, PLP variants were scattered across functional domains (Fig. 3).
Distribution of PLP variants in the functional domains of BRCA1 and BRCA2. Lollipop plot illustrating the frequency of PLP variants across the functional domain of BRCA1 [zinc finger, serine cluster, BRCA1 C-terminus (BRCT)] and BRCA2 [BRC repeats, DNA binding, and nuclear localization signals (NLS)]. The blue circles denote the frequency of PLPs found in the healthy individuals and the orange circles denote PLPs found in BRCA mutation related-cancer carriers. PLP variants were clustered in the BRC repeats and DNA binding domain in cancer patients in comparison to the healthy individuals, but not in NLS domain of BRCA2 and any functional domain of BRCA1
Geographically, we observed a significant agglomeration of healthy individuals carrying PLP variants in Yancheng City, with a prevalence of 3.39% (2/59) (Fischer’s Exact, P = 0.039). Yancheng City, with a population of 6.69 million, did not exhibit a significantly higher total BRCA variant load compared to other cities in China, leading to a unique geographical clustering of PLP variants in this city in the northeastern coastal region of China. We believe that a more extensive screening in local population is necessary to elucidate the interaction between genetics and the environment for cancer risk.
Case study of recurrent PLP variants’ carriers and incident cancer cases during follow-up
Among all the 2,080 normal individuals, we detected a recurrent pathogenic variant, BRCA1 c. 5470-5477del, in 2 independent subjects: a 51-year-old female and a 30-year-old female. This variant was also found in three of the 122 TNBC patients, specifically in a 42-year-old female, a 38-year-old female, and a 34-year-old female. Notably, the 51-year-old female carrying BRCA1 c. 5470-5474del variant developed bilateral breast lesions [Breast imaging-reporting and data system (BI-RAD) 4c, measuring 7 mm × 4 mm for the left and 8 mm × 6 mm for the right] during the follow-up ultrasound examination 16 months after her positive BRCA variant screening test. These breast lesions were surgically removed via lumpectomy and confirmed as regional invasive ductal carcinoma (basal-like) by pathology. No other sign of cancer was observed in this patient after the surgical operation.
Additionally, we identified one healthy individual and one TNBC patient sharing the BRCA2 c. 5682 C > G variant. Over the course of a 7-month follow-up, the 50-year-old female healthy carrier exhibited no clinical manifestation and yielded negative cancer examination results.
In the apparently healthy group, a 70-year-old male carrying BRCA2 c.3599_3600del nonsense variant was diagnosed with PDAC measuring 36 mm × 34 mm × 21 mm in the head-hook region 9 months after his BRCA testing. Furthermore, a 33-year-old female with BRCA2 c.3523 C > T variant developed invasive mucinous carcinoma in the left breast (measuring 22 mm × 15 mm × 10 mm) during her second annual examination (10 months) after BRCA mutation scanning. Additionally, we identified new tumors in two PLP variant-free individuals. One case involved a 59-year-old female diagnosed with left breast TNBC (measuring 24 mm × 15 mm × 15 mm) in the 11th month of her follow-up, and the other case was a 71-year-old male diagnosed with pancreatic body-tail PDAC (measuring 33 mm × 32 mm × 27 mm) in the 24th month of follow-up.
Similarity and difference of VUS between the general Chinese Han population and cancer cohorts
We also identified 131 VUS in the general Han Chinese population and 41 VUS in the cancer cohorts (Supplementary File 5). Among the 2,080 apparently healthy individuals, we observed 20 recurrent VUS. The most frequently occurring VUS was BRCA1 c.2726 A > T, found in 8 individuals from major areas of South China, including Shanghai (2 individuals), Zhejiang (2 individuals), Jiangsu (1 individual), Fujian Province (1 individual), and Guangdong Province (1 individual). This VUS was also observed in one patient bearing PDAC, a 71-year-old male from Jiangsu Province.
There is no significant VUS enrichment across the gene structures in the cancer group compared to the healthy group, which differs from the splicing mutations clustering in the tumor group among PLPs (P = 0.03). According to the ACGS classification criteria, we observed no significant enrichment of hot/warm variants in the tumor patients compared to the healthy individuals (Fischer’s exact, P = 0.96). The reclassification and scoring of VUS by computational prediction tools also revealed that the VUS harbored by PLP variant-free tumor patients and apparently healthy persons did not differ significantly according to the current machine learning algorithm including iMutant (P = 0.12), Mutation Taster (P = 0.20) and VEST (P = 0.81), EVE (P = 0.50) and REVEL (P = 0.17). This indicates that there should be more extensive research into the pathogenicity of VUS, for example, utilizing the large-scale clinical follow-up data.
In the cancer cohorts, we observed two recurrent VUS in BRCA2: c.2186T > C (found in a 69-year-old male from Zhejiang with mCRPC and a 74-year-old female from Shanghai with PDAC) and c.8971 C > T (found in a 28-year-old female from Hebei Province with TNBC and a 52-year-old male from Anhui Province with PDAC). The c. 2186T > C variant also appeared in three healthy individuals (a 76-year-old male from Shanghai, a 43-year-old male from Heilongjiang Province, and a 53-year-old male from Zhejiang), while c.8971 C > T was exclusive to cancer cases. Worth noting is that BRCA1 c. 3524 C > T was another cancer-specific VUS observed in a PLP-free 61-year-old female patient bearing primary PDAC and TNBC. We also identified BRCA1 c.548-15G > A in two unrelated healthy individuals, which was previously reported to induce the abnormal transcript splicing [38].
BRCA LGR in the general Chinese Han population and BRCA mutation-related cancer cohorts
LGR is another genomic contributor to BRCA inactivation beyond SNV and InDels. Using NGS data, we comprehensively analyzed CNV in BRCA1 and BRCA2 at amplicon level. After the MLPA experiment, we confirmed the presence of LGR in three healthy individuals, including two relatives with BRCA2 exon 22 to exon 24 deletion and one subject with BRCA2 exon 12 to exon 13 duplication. Interestingly, we did not identify any BRCA LGR among the 522 cancer patients. These evidence suggest the presence of BRCA LGRs in the general Chinese population, although the pathogenicity of these variations needs further validation with longer-term follow-up and broader population cohorts.
BRCA mutation screening identified individuals at BRCA mutation-related cancer risk in the general Chinese Han population
To assess whether BRCA screening can effectively discriminate individuals at elevated risk of BRCA mutation-related cancers from the general population, we conducted a prospective follow-up for tumor risk assessment in 1,005 individuals (11 BRCA PLP carriers and 994 BRCA PLP-free individuals) out of the apparently healthy group after their BRCA mutation test.
Throughout the 24-month follow-up period, we identified three new cases of BRCA mutation-related cancers (comprising 2 TNBC cases and 1 PDAC case) among the 11 BRCA PLP carriers. In the group of 994 BRCA PLP-free individuals, there were two new cases (1 TNBC and 1 PDAC). There were no statistically significant differences between the PLP carriers and PLP-free individuals in terms of gender distribution [36.36% (4/11) male (PLP carriers) vs. 56.74% (564/994) male (PLP-free), P = 0.23], age [42.91 ± 13.03 years (PLP carriers) vs. 48.47 ± 11.46 years (PLP-free), P = 0.11], or follow-up duration [median of 10 months (25th to 75th percentile: 7 to 20 months, PLP carriers) vs. median of 16 month (25th to 75th percentile: 10 to 19 months, PLP-free), P = 0.15]. Therefore, the relative risk for developing BRCA mutation-related cancer in the exposure to a positive BRCA germline mutation test is 135.55 (95% CI 25.07 to 732.88), with an absolute risk increasement = 27.07% (95% CI = 23.24–30.90%).
Discussion
BRCA germline mutation carriers face a high risk for BRCA mutation-related cancers. While BRCA variant screening effectively aids risk classification and prevention in those with familial history of breast/ovarian cancer [2, 39, 40], the mutational spectrum shifts across ethnicities [5, 6], causing debates about population-wide screening and its implementation [20].
Prior studies have explored the BRCA germline variants in the Chinese population, but challenges remain unaddressed: (1) most studies have concentrated on patients already diagnosed with BRCA mutation-related cancer [11, 14, 41]; (2) population-based studies on healthy individuals are regionally restricted (Taiwan [16], Macau [17], North China [18]; 3) there has been a lack of post-test follow-up to determine whether screening in the general population identifies high cancer risk individuals. The functional landscape of BRCA germline variation in the world’s largest genetic population, the Han Chinese, remains inadequately understood.
This study presents our experience in BRCA germline variant screening involving 2,080 apparently healthy population and 522 BRCA mutation-related cancer patients. It covered 33 of the 34 administrative regions in China, except Macau SAR, offering a diverse genetic representation of the Chinese Han Population. With a centralized recruitment, testing, and follow-up process, our pipeline ensured consistent and reliable conclusions.
We found an incidence of 0.53% (one in 189) for a Han Chinese to carry germline BRCA pathogenic or likely pathogenic variants. By consolidating our findings with those of previous studies in China, such as Dong et al. (0.53%, n = 11,386 normal Chinese) [18], Qin et al. (0.38%, n = 6,314 normal Macanese) [17], Chain et al. (0.53%, n = 1,517 Taiwanese) [16], Liu et al. (1.10%, n = 6,434 normal control for breast cancer) [9], Lang et al. (0.38%, n = 1,043 normal control for breast cancer) [14], and Li et al. (0.34%, n = 1,763 normal control for ovarian cancer), we estimated a 0.52% (95% CI = 0.30–0.84%) prevalence of deleterious BRCA mutations in the Chinese Han. This is lower than the established rate in Ashkenazi Jews (2%) [42], similar to the American and British populations (0.5%) [43], and slightly higher than other East Asian populations, including Japanese and Korean (0.2%) [44]. The consistent variant frequency across various Chinese studies underscores the stable baseline of BRCA germline variations in this demographic. However, we also discovered significant regional differences in the mutational spectrum. For example, the founder mutation BRCA1 c.5470_5477del is specifically harbored by the North Han in our study (0.44% in healthy individuals and 5.89% in cancer patients), and this variant has not been reported in previous BRCA variant screening studies conducted in the south region of China [45,46,47]. Furthermore, the PLP variants found in our healthy group, including BRCA1 c.2934, c.5521del, c.869del, and BRCA2 c.3523 C > T, c.8650del, c.9753del have not been reported in previous screenings of the normal Chinese population [9, 10, 14, 16,17,18].
Of note, all identified PLP carriers denied a family history of cancer during the pre-test genetic counseling, and there were no serological or radiological indications of tumors. These oversights emphasized the limitations of family history-based screening strategy: it mandates the presence of a family member with cancer diagnosis and a well-documented family history of disease.
The mutational spectrum of PLP variants differs between healthy individuals and cancer patients. Among the 11 BRCA1 PLPs identified in our study, only one (c. 5470_5477del) was common to both healthy individuals and cancer patients. Similarly, these two groups shared only one of the 17 BRCA2 PLPs (c. 5682 C > G). However, the presence of these PLPs in healthy individuals does not negate their pathogenicity. Actually, three out of the 11 individuals carrying these PLPs developed BRCA mutation-related cancer during follow-up. For instance, BRCA1 c.5470_5477del showed a relatively high prevalence (0.26%, 3/1151) in the North Han and demonstrated penetrance for TNBC [35]. In addition, more efforts should be encouraged on further categorizing the pathogenicity of VUS, such as the recurrent BRCA2 c.8971 C > T in the cancer cohort and the potential splicing abnormalities causing BRCA1 c.548-15G > A in the healthy group. Long-term phenotypic follow-up will provide evidence-based medicine level insight beyond the current machine-learning approach.
In contrast to the aggregation of PLP variants within the functional domains of BRCA (BRCA2 BRC repeat and DNA-binding) in cancer patients [48, 49], we observed a uniformed distribution of PLPs across BRCA1 and BRCA2 sequence in the healthy individuals. This supports the hypothesis that BRCA pathogenic variants originated relatively recently in human history [28]; however, further disease penetrating restricted the complexity of the variants into a specific genomic region. These findings highlight the necessity of employing NGS for germline mutation screening in BRCA.
Apart from SNV and small insertion/deletion, we observed two types of LGRs in three out of the 2,080 healthy individuals. These included two individuals with kinship harboring the same exon 22 to exon 24 deletion in BRCA2 [50]. Notably, LGRs were not observed in the 522 cancer patients. All three individuals with LGRs have not shown any sign of developing malignancies so far, even after follow-ups at the ages of 52, 55, and 78. This explains the relatively low penetrance of LGR (~ 1%) in BRCA mutation-related cancers in China [51, 52], compared to European patients [53, 54]. However, long-term follow-up beyond the 24-month shall be encouraged to elucidate the pathogenicity of these structure variants. It also highlights the need for a sensitive and specific algorithm for LGR calling using NGS data.
Our follow-up on 1,005 healthy Chinese Han individuals showed that those with positive BRCA variant tests had significantly increased BRCA mutation-related cancer risks (RR = 135.55, 95% CI 25.07 to 732.88) after accounting for potential confounders, including age, gender, and duration of follow-up. While the relatively small sample size in the PLP carrier group might cause overestimation, it highlights the value of BRCA mutation screening in the general Chinese Han population.
There is emerging evidence that population-wide screening is a better approach for the prevention of BRCA mutation-related cancer since family history-based screening misses a significant portion of individuals carrying the BRCA variant [7]. Considering the relatively high prevalence and mutational profile background in this context, the unique “small family” structure within the major Chinese populations, and the widespread culture of “medical stigmatization” in East Asia, we suggest broader BRCA variant screening, accompanied by detailed comprehensive genetic counseling.
One limitation of this study is the relatively short follow-up period, which may not adequately reflect the relative risk of cancer development in PLP carriers compared to PLP-free individuals. Additionally, since the participation of follow-up is voluntary other than mandatory, healthy individuals with the PLP-free results from BRCA mutation screening may have limited willingness to participate in the follow-up, leading to a 50% follow-up rate in this study. Furthermore, although our study included 2,080 healthy individuals, the regional sampling bias limited our findings primarily to the East Han population, and does not fully represent the situation within the 1.4 billion Chinese Han population. Moreover, While the single-center design enhanced the comparability of the testing and follow-up data, it also introduced sampling bias and other unexpected confounders. Therefore, a multi-center prospective study is encouraged to elucidate the medical benefits of population-based screening of BRCA germline variants in the Chinese Han population. Further cost-effectiveness studies comprehending the balance between variant screening and financial expenditure [55, 56], and psychosocial studies [57, 58] on the impact of genetic test results, will facilitate devising the optimal screening strategy in the Chinese Han population.
Conclusion
By integrating NGS data from 2,080 apparently healthy individuals, we have characterized the genetic landscape of germline BRCA variants, including SNVs, small InDels and LGRs, in the Chinese Han, with a special focus on the East Han subpopulation. The mutational spectrums are of significant difference between the healthy individuals and cancer patients. Furthermore, we conducted a short-term follow-up involving 1,005 individuals from the healthy group, confirming that individuals identified as PLP carriers by population-based screening face a significantly elevated risk of developing BRCA mutation-related cancer compared to those without PLPs.
Our study highlights the utility of BRCA germline variant screening for risk stratification and early cancer detection in the apparently healthy Chinese Han individuals. We advocate for multi-center prospective studies to assess the medical benefits of population-based BRCA germline variant screening compared to conventional family history-based screening in the Chinese Han population. Additionally, we anticipate that our research, along with investigations into financial considerations and psychosocial impact of genetic test results, will contribute to the development of an optimal screening strategy for the Chinese Han population.
Data availability
The datasets used and/or analysed during the current study are available in the main text and supplementary files of the manuscript.
Abbreviations
- BRCA:
-
BRCA1/2
- SNV:
-
Single nucleotide variation
- InDel:
-
Insertion and deletion
- LGR:
-
Large genomic rearrangement
- NGS:
-
Next-generation sequencing
- TNBC:
-
Triple-negative breast cancer
- mCRPC:
-
Metastatic castration-resistant prostate cancer
- PDAC:
-
Pancreatic ductal adenocarcinoma
- HGOC:
-
High-grade ovarian cancer
- ACMG:
-
American College of Medical Genetics
- CNV:
-
Copy number variation
- PLP:
-
Pathogenic/likely pathogenic
- ACGS:
-
Association for Clinical Genomic Science
- VUS:
-
Variant of unknown significance
- BLB:
-
benign/likely benign
- BI-RAD:
-
Breast imaging-reporting and data system
- RR:
-
Relative risk
References
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–20.
Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, Senkus E, Committee EG. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103–10.
Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Garber JE, et al. NCCN guidelines insights: Genetic/Familial High-Risk Assessment: breast, ovarian, and pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18(4):380–91.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–43.
Rebbeck TR, Friebel TM, Friedman E, Hamann U, Huo D, Kwong A, Olah E, Olopade OI, Solano AR, Teo SH, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39(5):593–620.
Bhaskaran SP, Chandratre K, Gupta H, Zhang L, Wang X, Cui J, Kim YC, Sinha S, Jiang L, Lu B, et al. Germline variation in BRCA1/2 is highly ethnic-specific: evidence from over 30,000 Chinese hereditary breast and ovarian cancer patients. Int J Cancer. 2019;145(4):962–73.
Manchanda R, Sun L, Patel S, Evans O, Wilschut J, De Freitas Lopes AC, Gaba F, Brentnall A, Duffy S, Cui B et al. Economic evaluation of Population-based BRCA1/BRCA2 mutation testing across multiple countries and Health systems. Cancers (Basel) 2020, 12(7).
Kemp Z, Turnbull A, Yost S, Seal S, Mahamdallie S, Poyastro-Pearson E, Warren-Perry M, Eccleston A, Tan MM, Teo SH, et al. Evaluation of Cancer-based Criteria for Use in Mainstream BRCA1 and BRCA2 genetic testing in patients with breast Cancer. JAMA Netw Open. 2019;2(5):e194428.
Liu Y, Wang H, Wang X, Liu J, Li J, Wang X, Zhang Y, Bai Z, Zhou Q, Wu Y, et al. Prevalence and reclassification of BRCA1 and BRCA2 variants in a large, unselected Chinese Han breast cancer cohort. J Hematol Oncol. 2021;14(1):18.
Li A, Xie R, Zhi Q, Deng Y, Wu Y, Li W, Yang L, Jiao Z, Luo J, Zi Y, et al. BRCA germline mutations in an unselected nationwide cohort of Chinese patients with ovarian cancer and healthy controls. Gynecol Oncol. 2018;151(1):145–52.
Yin L, Wei J, Lu Z, Huang S, Gao H, Chen J, Guo F, Tu M, Xiao B, Xi C, et al. Prevalence of germline sequence variations among patients with pancreatic Cancer in China. JAMA Netw Open. 2022;5(2):e2148721.
Zhu Y, Wei Y, Zeng H, Li Y, Ng CF, Zhou F, He C, Sun G, Ni Y, Chiu PKF, et al. Inherited mutations in Chinese men with prostate Cancer. J Natl Compr Canc Netw. 2021;20(1):54–62.
Lei H, Zhang M, Zhang L, Hemminki K, Wang XJ, Chen T. Overview on population screening for carriers with germline BRCA mutation in China. Front Oncol. 2022;12:1002360.
Lang GT, Shi JX, Hu X, Zhang CH, Shan L, Song CG, Zhuang ZG, Cao AY, Ling H, Yu KD, et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer. 2017;141(1):129–42.
Cao Y, Li L, Xu M, Feng Z, Sun X, Lu J, Xu Y, Du P, Wang T, Hu R, et al. The ChinaMAP analytics of deep whole genome sequences in 10,588 individuals. Cell Res. 2020;30(9):717–31.
Chian J, Sinha S, Qin Z, Wang SM. BRCA1 and BRCA2 variation in Taiwanese General Population and the Cancer Cohort. Front Mol Biosci. 2021;8:685174.
Qin Z, Kuok CN, Dong H, Jiang L, Zhang L, Guo M, Leong HK, Wang L, Meng G, Wang SM. Can population BRCA screening be applied in non-ashkenazi jewish populations? Experience in Macau population. J Med Genet. 2021;58(9):587–91.
Dong H, Chandratre K, Qin Y, Zhang J, Tian X, Rong C, Wang N, Guo M, Zhao G, Wang SM. Prevalence of BRCA1/BRCA2 pathogenic variation in Chinese Han population. J Med Genet. 2021;58(8):565–9.
Yurgelun MB, Hiller E, Garber JE. Population-wide screening for germline BRCA1 and BRCA2 mutations: too much of a good thing? J Clin Oncol. 2015;33(28):3092–5.
Ficarazzi F, Vecchi M, Ferrari M, Pierotti MA. Towards population-based genetic screenings for breast and ovarian cancer: a comprehensive review from economic evaluations to patient perspectives. Breast. 2021;58:121–9.
Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Goggins M, Hutton ML, et al. Genetic/Familial High-Risk Assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77–102.
Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181–5.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of Tumours of the urinary system and male genital organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–19.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, Board WHOCTE. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8.
McCluggage WG, Singh N, Gilks CB. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology. 2022;80(5):762–78.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Chen D. [A Chinese interpretation for the ACGS Best Practice guidelines for variant classification in Rare Disease 2020]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2023;40(8):915–21.
Li J, Zhao B, Huang T, Qin Z, Wang SM. Human BRCA pathogenic variants were originated during recent human history. Life Sci Alliance 2022, 5(5).
Poon KS. In silico analysis of BRCA1 and BRCA2 missense variants and the relevance in molecular genetic testing. Sci Rep. 2021;11(1):11114.
Capriotti E, Fariselli P, Casadio R. I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33(Web Server issue):W306–310.
Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–6.
Carter H, Douville C, Stenson PD, Cooper DN, Karchin R. Identifying mendelian disease genes with the variant effect scoring tool. BMC Genomics. 2013;14(Suppl 3):S3.
Frazer J, Notin P, Dias M, Gomez A, Min JK, Brock K, Gal Y, Marks DS. Disease variant prediction with deep generative models of evolutionary data. Nature. 2021;599(7883):91–5.
Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, Musolf A, Li Q, Holzinger E, Karyadi D, et al. REVEL: an Ensemble Method for Predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99(4):877–85.
Meng H, Yao L, Yuan H, Xu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, et al. BRCA1 c.5470_5477del, a founder mutation in Chinese Han breast cancer patients. Int J Cancer. 2020;146(11):3044–52.
Deng M, Chen HH, Zhu X, Luo M, Zhang K, Xu CJ, Hu KM, Cheng P, Zhou JJ, Zheng S, et al. Prevalence and clinical outcomes of germline mutations in BRCA1/2 and PALB2 genes in 2769 unselected breast cancer patients in China. Int J Cancer. 2019;145(6):1517–28.
Wei Y, Wu J, Gu W, Qin X, Dai B, Lin G, Gan H, Freedland SJ, Zhu Y, Ye D. Germline DNA repair Gene Mutation Landscape in Chinese prostate Cancer patients. Eur Urol. 2019;76(3):280–3.
Dong Z, Wang Y, Zhang J, Zhu F, Liu Z, Kang Y, Lin M, Shi H. Analyzing the effects of BRCA1/2 variants on mRNA splicing by minigene assay. J Hum Genet. 2023;68(2):65–71.
Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast Cancer: a review. JAMA Surg. 2017;152(6):589–94.
Sessa C, Balmaña J, Bober SL, Cardoso MJ, Colombo N, Curigliano G, Domchek SM, Evans DG, Fischerova D, Harbeck N, et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann Oncol. 2023;34(1):33–47.
Wu X, Wu L, Kong B, Liu J, Yin R, Wen H, Li N, Bu H, Feng Y, Li Q, et al. The First Nationwide Multicenter Prevalence Study of Germline BRCA1 and BRCA2 mutations in Chinese ovarian Cancer patients. Int J Gynecol Cancer. 2017;27(8):1650–7.
Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi jews. Am J Hum Genet. 1999;64(4):963–70.
Manchanda R, Blyuss O, Gaba F, Gordeev VS, Jacobs C, Burnell M, Gan C, Taylor R, Turnbull C, Legood R, et al. Current detection rates and time-to-detection of all identifiable BRCA carriers in the Greater London population. J Med Genet. 2018;55(8):538–45.
Maxwell KN, Domchek SM, Nathanson KL, Robson ME. Population frequency of germline BRCA1/2 mutations. J Clin Oncol. 2016;34(34):4183–5.
Wang Q, Wu H, Lan Y, Zhang J, Wu J, Zhang Y, Li L, Liu D, Zhang J. Changing patterns in clinicopathological characteristics of breast Cancer and prevalence of BRCA mutations: analysis in a rural area of Southern China. Int J Gen Med. 2021;14:7371–80.
Luo Y, Wu H, Huang Q, Rao H, Yu Z, Zhong Z. The features of BRCA1 and BRCA2 germline mutations in Hakka Ovarian Cancer patients: BRCA1 C.536 A > T maybe a founder mutation in this Population. Int J Gen Med. 2022;15:2773–86.
Shui L, Li X, Peng Y, Tian J, Li S, He D, Li A, Tian B, Li M, Gao H, et al. The germline/somatic DNA damage repair gene mutations modulate the therapeutic response in Chinese patients with advanced pancreatic ductal adenocarcinoma. J Transl Med. 2021;19(1):301.
Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78.
Heramb C, Wangensteen T, Grindedal EM, Ariansen SL, Lothe S, Heimdal KR, Maehle L. BRCA1 and BRCA2 mutation spectrum - an update on mutation distribution in a large cancer genetics clinic in Norway. Hered Cancer Clin Pract. 2018;16:3.
Hua D, Tian Q, Wang X, Bei T, Cui L, Zhang B, Bao C, Bai Y, Zhao X, Yuan P. Next-generation sequencing based detection of BRCA1 and BRCA2 large genomic rearrangements in Chinese cancer patients. Front Oncol. 2022;12:898916.
Cao WM, Zheng YB, Gao Y, Ding XW, Sun Y, Huang Y, Lou CJ, Pan ZW, Peng G, Wang XJ. Comprehensive mutation detection of BRCA1/2 genes reveals large genomic rearrangements contribute to hereditary breast and ovarian cancer in Chinese women. BMC Cancer. 2019;19(1):551.
Su L, Zhang J, Meng H, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y. Prevalence of BRCA1/2 large genomic rearrangements in Chinese women with sporadic triple-negative or familial breast cancer. Clin Genet. 2018;94(1):165–9.
Riahi A, Chabouni-Bouhamed H, Kharrat M. Prevalence of BRCA1 and BRCA2 large genomic rearrangements in Tunisian high risk breast/ovarian cancer families: implications for genetic testing. Cancer Genet. 2017;210:22–7.
Bozsik A, Pocza T, Papp J, Vaszko T, Butz H, Patocs A, Olah E. Complex characterization of Germline large genomic rearrangements of the BRCA1 and BRCA2 genes in high-risk breast Cancer patients-Novel variants from a large National Center. Int J Mol Sci 2020, 21(13).
Manchanda R, Legood R, Burnell M, McGuire A, Raikou M, Loggenberg K, Wardle J, Sanderson S, Gessler S, Side L, et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J Natl Cancer Inst. 2015;107(1):380.
Manchanda R, Patel S, Gordeev VS, Antoniou AC, Smith S, Lee A, Hopper JL, MacInnis RJ, Turnbull C, Ramus SJ, et al. Cost-effectiveness of Population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in Unselected General Population women. J Natl Cancer Inst. 2018;110(7):714–25.
Halbert CH, Stopfer JE, McDonald J, Weathers B, Collier A, Troxel AB, Domchek S. Long-term reactions to genetic testing for BRCA1 and BRCA2 mutations: does time heal women’s concerns? J Clin Oncol. 2011;29(32):4302–6.
Watson M, Foster C, Eeles R, Eccles D, Ashley S, Davidson R, Mackay J, Morrison PJ, Hopwood P, Evans DG, et al. Psychosocial impact of breast/ovarian (BRCA1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Br J Cancer. 2004;91(10):1787–94.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China [82272415 to Z.W], Shanghai Municipal Health Commission (grant number 2022YQ045 to Z.W.), Shanghai “Rising Stars of Medical Talents”–Clinical Laboratory Practitioner Program (grant number 2022-065 to Z.W), and the Innovation Group Project of Shanghai Municipal Health Commission (grant number 2019CXJQ03 to M.G.).
Author information
Authors and Affiliations
Contributions
Zhiyuan Wu and Ming Guan organized the project and wrote the paper. Zhiyuan Wu, Yiting Jin, Qingyun Zhang and Can Yang analyzed and interpreted the data. Qingyun Zhang, Xinju Zhang, Xuemei Tang and Yanli Chen performed the experiment. Yiting Jin, Haowen Jiang, Xiaoyi Wang, Xinli Zhou, Feng Yu, Bing Wang recruited patients, provided clinical information, obtained informed consent. All authors contributed to and approved the final manuscript. The work reported in the paper has been performed by the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Huashan Hospital of Fudan University (2023 − 812). Written informed consent was received from all participants.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, Z., Zhang, Q., Jin, Y. et al. Population-based BRCA germline mutation screening in the Han Chinese identifies individuals at risk of BRCA mutation-related cancer: experience from a clinical diagnostic center from greater Shanghai area. BMC Cancer 24, 411 (2024). https://doi.org/10.1186/s12885-024-12089-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12089-w