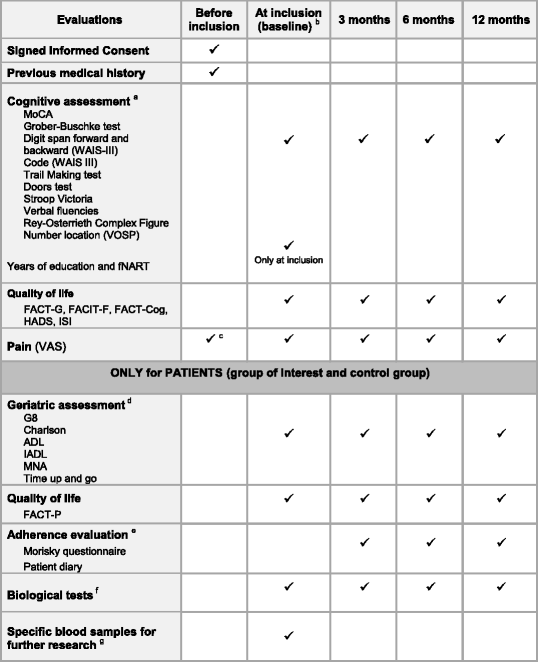
- MoCA Montreal Cognitive Assessment, WAIS Wechsler Adult Intelligence Scale, VOSP Visual Object and Space Perception Battery, fNART French National Adult Reading Test, ISI Insomnia Severity Index, VAS Visual Analog Scale, ADL Activities of Daily Living, IADL Instrumental Activities of Daily Living, MNA Mini-Nutritional Assessment
- aCognitive assessment will be performed by neuropsychologists
- bFor group of interest patients: before the start of the treatment or within 15 days after the start of treatment by abiraterone acetate or enzalutamide
- cHad to be ≤3 on the 0–10 pain VAS scale to meet with inclusion pain criteria
- dGeriatric assessment will be performed by a study nurse specialized in geriatric
- eAdherence evaluation will be performed only in group of interest patients
- fAt each time: CBC, platelets, albumin, CRP, prealbumin, iron, ferritin, transferrin, creatinin, sodium, potassium, ALT, AST, GGT, ALP, total bilirubin, TSH, T4, testosterone. At inclusion only: cortisol (at 8 h AM, fasting)
- g1 EDTA (5 ml), 1 dry tube with gel (5 ml) and 1 dry tube without gel (5 ml)
